Chemistry
Get insights from 6.9k questions on Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
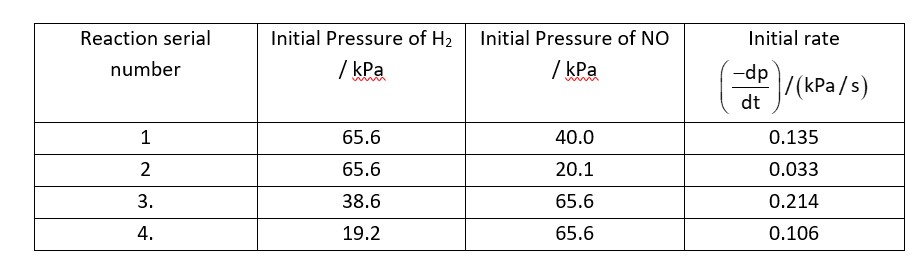
On decreasing pressure of NO by a factor of '2' the rate of reaction decreases by a factor of '4'
Order of reaction w.r.t 'NO' = 2
New answer posted
5 months agoContributor-Level 10
Meq of K2Cr2O7 = Meq of Fe2+
-> (Molarity * Volume * nf) of K2Cr2O7 = (molarity * volume * nf) of Fe2+
->0.02 * 20 * 6 = M * 10 * 1
M = 0.24 M
Molarity = 24 * 10-2 M
New answer posted
5 months agoBeginner-Level 5
The central atom of nitrogen has 5 valence electrons as per the electronic configuraion. During the formation of , 3 valence electrons forms three sigma bonds with hydrogen and one lone electron pair is left.
- The steric number of the ammonia molecule: SN=3 bonds +1 lone pair total electron domains.
- As per the steric number, there is hybridisation in ammonia.
- The lone pair causes repulsion, which leads to a trigonal pyramidal geometry with bond angles of .
New answer posted
5 months agoContributor-Level 10
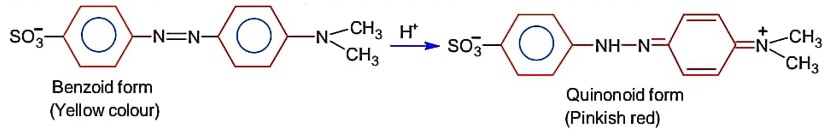
For an acid- base titration, Methly orange exist at end point as quinonoid form.
New answer posted
5 months agoContributor-Level 10
In sucrose, glycosidic linkage is between C1 of α-glucose and C2 of β-fructose.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers