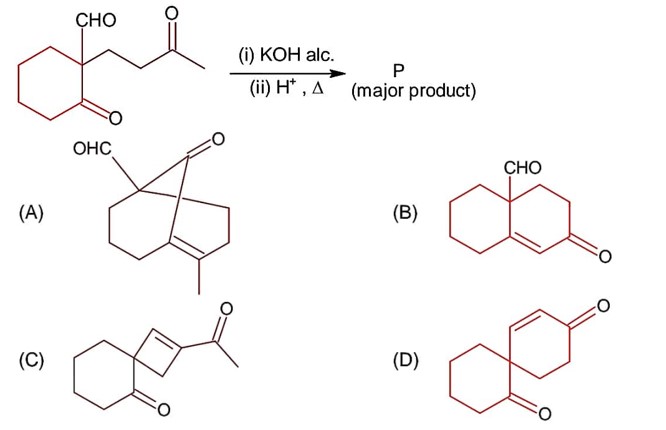
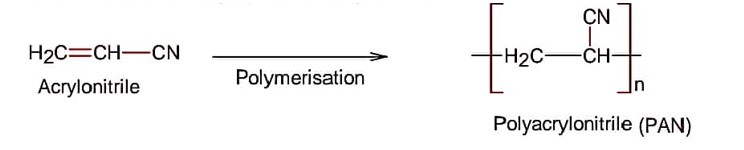
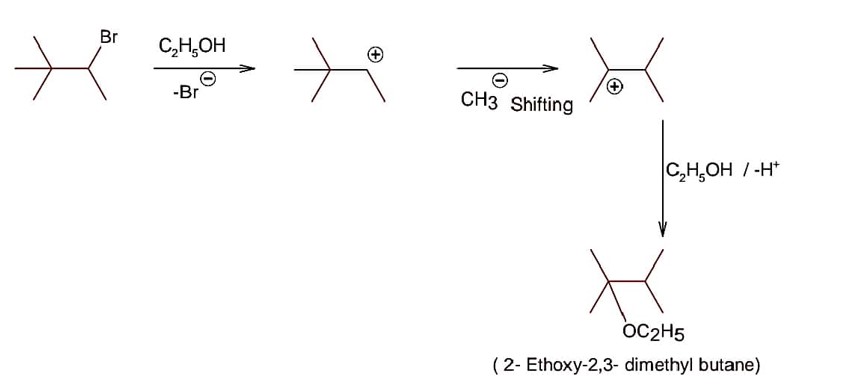
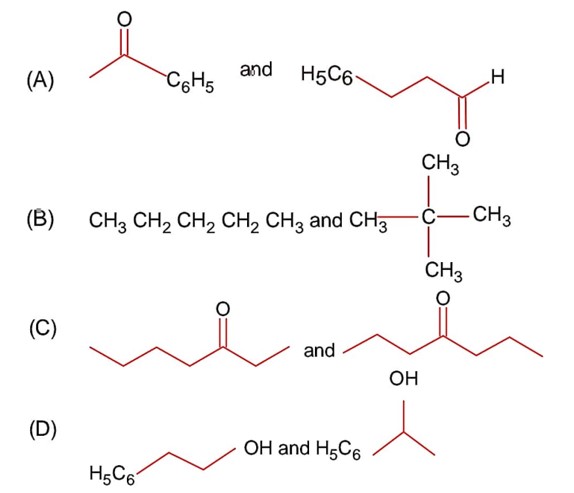
Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
Wt of Cl- in 100 ml = 1.8 * 10-3 gm
Mol. of Cl- in 100 ml =
i.e. 0.507 milli mole in one lit required in one hr.
Coagulation value = (millimole/lit) required in one hr = 0.507
= 1 (the nearest integer)
New answer posted
5 months agoContributor-Level 10
0.15 gm (organic compounds)
0.2397 gm
Weight of Br in AgBr =
% Br in compound =
New question posted
5 months agoNew answer posted
5 months agoContributor-Level 10
Due to the larger acid dissociation constant (Ka) sulphuric acid acts as an acid and HNO3 acts as a base.
New answer posted
5 months agoContributor-Level 10
Due to the absence of acidic hydrogen in But – 2-yne, metallic sodium does not react with this.
New answer posted
5 months agoContributor-Level 10
In metamers, distribution of alkyl groups are changed with respect to polyvalent functional groups.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers