Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
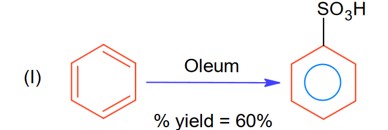
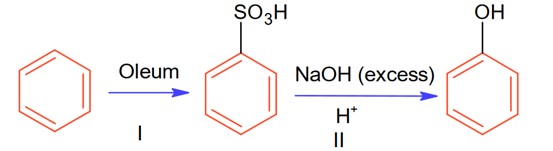
Let initial moles of reactant taken = n. Total moles obtained for benzene sulphonic acid = 0.6 n (with % yield = 60%)
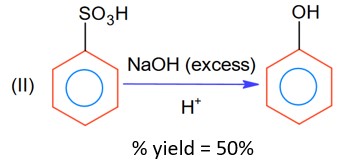
Moles of benzene sulphonic acid before reaction II = 0.6n
Moles obtained for phenol (with % yield 50%) = 0.6 * 0.5 n = 0.3 n
So, overall % age yield of complete reaction =
New answer posted
5 months agoContributor-Level 10
Oxidation state of carbon changes from +3 to +4
change in O. S is 1
New answer posted
5 months agoContributor-Level 10
On decreasing pressure of NO by a factor of '2' the rate of reaction decreases by a factor of '4'
Order of reaction w.r.t 'NO' = 2
New answer posted
5 months agoContributor-Level 10
Meq of K2Cr2O7 = Meq of Fe2+
(Molarity * Volume * nf) of K2Cr2O7 = (molarity * volume * nf) of Fe2+
0.02 * 20 * 6 = M * 10 * 1
M = 0.24 M
Molarity = 24 * 10-2 M
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers