Physics Atoms
Get insights from 74 questions on Physics Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Linear momentum refers to the motion in a straight line, from one point to another. And angular momentum is the rotational equivalent of linear momentum. Since the electrons move in circular tracks or orbitals, the angular momentum is used to calculate the movement of these electrons.
New answer posted
3 months agoContributor-Level 10
This theory simply stated that there exist stationary orbits around the nucleus in which electrons rotate without dissipating any type of energy. And if the electrons try to change their orbits, then there will be a gain or loss of energy depending on the situation.
New question posted
3 months agoNew answer posted
3 months agoContributor-Level 10
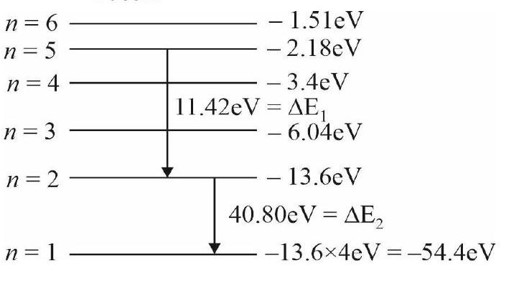
The spectrum of H-atom is formulated and drawn by Bohr's model. These spectrums are line spectrum. Lyman series lies in UV region, Balmer Partly lies UV and partly in the visible region and Paschen series lies in the infrared region.
New answer posted
3 months agoContributor-Level 10
The spectrum of H-atom is formulated and drawn by Bohr's model. These spectrums are line spectrum. Lyman series lies in UV region, Balmer Partly lies UV and partly in the visible region and Paschen series lies in the infrared region.
New answer posted
3 months agoContributor-Level 10
With the help of conservation of linear momentum, we can write
New answer posted
4 months agoContributor-Level 10
(hc/λ) = 13.6Z² (1/1² - 1/4²) = 13.6Z² (3/4) ; [λ = (4/3)k]
β line of Paschen
(hc/λ') = 13.6Z² (1/9 - 1/25)
λ = k * (9*25/16) ; λ' = (3/4)λ * (9*25/16) = (3³*5²/2? )λ
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers