Physics Atoms
Get insights from 74 questions on Physics Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Physics Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
E_K - E_L = hc/λ_Kα
E_K - E_L = (4.14*10? ¹? * 3*10? )/0.071*10? = 17500 eV = 17.5 keV.
E_L = E_K - 17.5 = 27.5 - 17.5 = 10 keV.
New answer posted
4 months agoContributor-Level 10
Energy of electron = 3 eV
It forms H atom in n=3 state. Energy released E = 3 - (-13.6/9) = 3 + 1.51 = 4.51 eV.
Photon Energy = 4.51 eV
Threshold energy = hc/λ = 12400eVÅ / 4000Å = 3.1 eV.
kE_max = 4.51 - 3.1 = 1.41 eV
New answer posted
4 months agoContributor-Level 10
r = 0.5Å = 0.5*10? ¹? m
v = 2.2*10? m/s
I =?
t = 2πr/v
I = e/t = ev/2πr = (1.6*10? ¹? * 2.2*10? )/ (2 * 22/7 * 0.5*10? ¹? )
I ≈ 1.12*10? ³ A = 1.12 mA = 112 * 10? ² mA
New answer posted
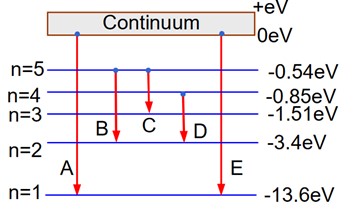
4 months agoContributor-Level 10
A : Series limit of Lyman series
B : Third line of Balmer series
C : Second line of Paschen series
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
Lyman Series nf = 1, ni = 2, 3.
Paschen series nf = 3, ni = 4, 5, 6 -
New answer posted
4 months agoContributor-Level 10
(1) n1 = 3, n2 = 2
(2) n1 = 4, n2 = 3
(3) n1 = 2, n2 = 1
(4) n1 = 5, n2 = 4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers