Chemistry NCERT Exemplar Solutions Class 11th Chapter Six
Get insights from 63 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Six, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Six
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
The correct option is B
The balanced equation for the combustion of methane is:
CH4 (g)+2O2 (g)→CO2 (g)+2H2O (l)
Here, Δng=1−3=−2
ΔH
=ΔU
+ΔngRT
ΔH
=−393−2RT
∴ΔH
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iii)
Standard enthalpy of combustion is defined as the enthalpy change per mole (or per unit amount) of a substance when it undergoes combustion and all the reactants and products being in their standard states at the specified temperature.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
Specific heat capacity is the quantity of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (or 1 Kelvin). That is why it is an intensive property which does not depend on mass.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (i)
Variables like p, V, T are called state variables or state functions because their values depend only on the state of the system and not on how it is reached.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
The presence of reactants in a closed vessel made of conducting material e.g., copper or steel is an example of a closed system.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Thermodynamics is not concerned about how and at what rate these energy transformations are carried out but is based on initial and final states of a system undergoing the change. Laws of thermodynamics apply only when a system is in equilibrium or moves from one equilibrium state to another equilibrium state.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
We know that the amount of work done =-pext? V
On substituting the values in the formula, we get,
-2bar* (50-10)L=-80Lbar
According to the described problem,1 LBar = 100J
Therefore, -80 L bar= (-80*100)= -8000J
= -8kJ, which is the amount of work done
The significance of the negative sign states that the work is done on the surroundings of the system. In the case of reversible expansion, the work done will be more.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
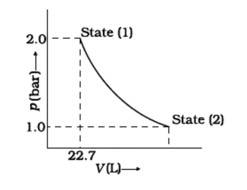
We can conclude from the figure that this change is a reversible change.
Now,
W= -2.303nRT log
But, p1V1 = p2V2 = = = =2
W= -2.303nRT log
= -2.303 *8.314*1*298*log2
= -2.303 *8.314*298*0.3010J
= -1717.46J
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
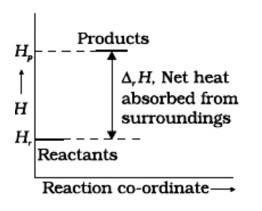
No, for the state of spontaneity, the enthalpy change is not the only criteria. Entropy also needs to be taken into account here.
New answer posted
7 months agoContributor-Level 10
This is a Short Answer Type Questions as classified in NCERT Exemplar
Throwing a stone from ground to roof
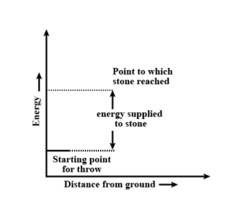
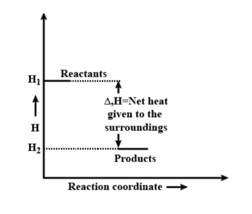
b) the reaction involved is a process where the energy decreases after the reaction. It can be represented as:In process b), potential energy/enthalpy change is a contributing factor to the spontaneity.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers