Chemistry NCERT Exemplar Solutions Class 11th Chapter Six
Get insights from 63 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Six, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Six
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
option (i)
Explanation: The entropy of a liquid reduces as it crystallises. Because the molecules are more organised in crystalline form.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: (ii)
Explanation: The energy factor for a spontaneous process should be favourable (i.e., -ve) and the randomness should be positive.
New answer posted
6 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
option (ii)
Explanation: The enthalpy of the reactants is always greater than the enthalpy of the product in a combustion reaction.
New answer posted
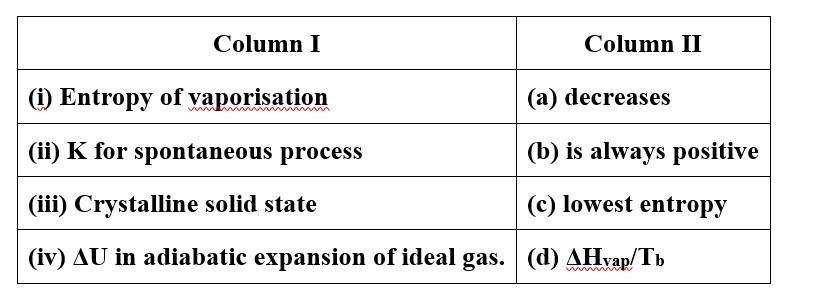
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (b), (d)
(ii)- (b)
(iii)- (c)
(iv)- (a)
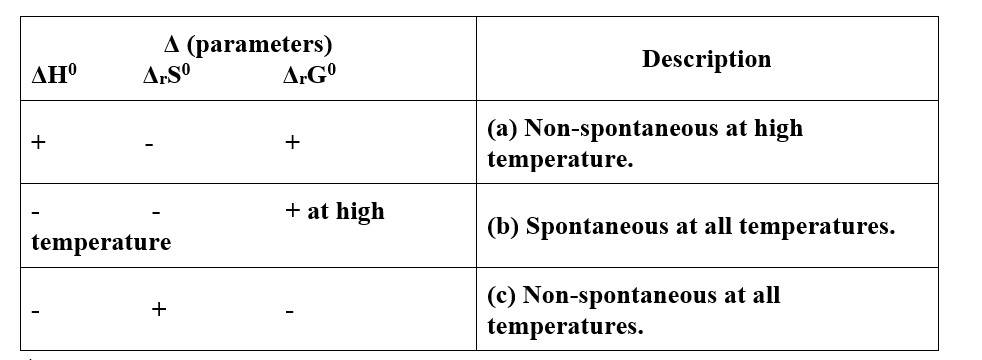
New answer posted
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (c)
(ii)- (a)
(iii)- (b)
New answer posted
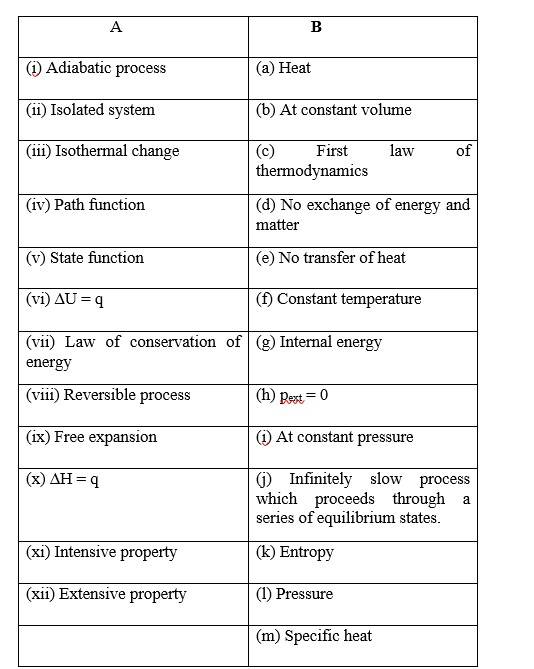
6 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
(i) (b)
(ii) (c)
(iii) (a)
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (i) and (iii)
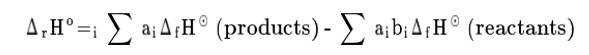
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iv)
For
isothermal reversible change
q= -w = nRTln =2.303nRTlog
= = =2
For
isothermal expansion of ideal gases, ? U = 0
Since,
temperature is constant this means there is no change in internal energy.
New answer posted
6 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
option (iii) and (iv)
Gas expands to fill the available space spontaneously, and burning of carbon to carbon dioxide is spontaneous
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers