Chemistry NCERT Exemplar Solutions Class 12th Chapter Fifteen
Get insights from 85 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Fifteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Fifteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Monomers used in addition to polymerization through free radical pathway should be very pure because even the traces of impurities acts like inhibitors. It leads to the formation of polymers with a shorter chain lengths.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Proteins have some similar structural similarities with synthetic polyamides. Proteins and polyamides both contain amide linkage.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Urea-formaldehyde resin is used in laminated sheets. The monomer involved in the formation of urea formaldehyde resin is urea and formaldehyde.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Polymers that possess amide linkage are termed as nylon. Fibers have a strong intermolecular force of attraction like hydrogen bonding. It leads to the close packing of the structure which imparts the crystalline nature.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Free radicals are generated from benzoyl peroxide. Alkanes, dienes are polymerized in the presence of free radical inhibitors like benzoyl peroxide, acetyl peroxide. For example, benzoyl peroxide inhibitor is used for the polymerization of ethene to polythene. First, phenyl peroxide radicals are formed which are added to the double bond of ethene generating a new and larger free radical.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
LDP: The polymerization of ethene under high pressure and temperature in the presence of peroxide catalyst gives low-density polythene. It is obtained through free radical addition and has a high branched structure. It is chemically inert and is a poor conductor of electricity. It is used for manufacturing toys, bottles.
HDP: When addition polymerization of ethene takes place in a hydrocarbon solvent in the presence of a catalyst such as triethylaluminium and Ziegler-Natta catalyst at high temperature and pressure then high-density polythene is formed. It consists of li
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Cis-polyisoprene has a coiled structure which consists of various chains held together by weak Van there Waals force of attraction. It can be stretched like a spring and thus, has elastic properties.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
A vulcanisation process is used to improve the physical properties of natural rubber. This process consists of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between 373 K to 415 k. Sulphur forms cross links at the reactive sites of double bonds during vulcanization, stiffening the rubber.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
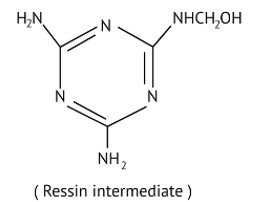
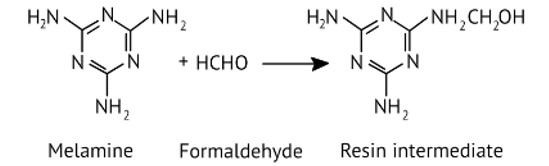
Melamine formaldehyde polymer is formed by the condensation polymerization of melamine and formaldehyde. This is resin intermediate.
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Yes, nucleic acids, proteins, and starch can be considered step growth polymers because this type of polymerization involves a condensation reaction between two monomers. Loss of simple molecules such as water, alcohol results in this type of polymerization and it results in the formation of high molecular mass polymers.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers