Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Get insights from 126 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Five, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
PV = nmixRT
Let moles of He is x
Moles of H2 = 3 – x
4x + 2 (3 – x) = 10
x = 2 mol
Mass of He = 8 gm
New answer posted
5 months agoContributor-Level 10
the finest gold sol is red in colour, as the size of particle increases, it appears purple then blue and finally gold. The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles. The wavelength of light further depends on size and nature of the particles. Hence, both assertion and reason are true and reason is the correct explanation of assertion.
New answer posted
6 months agoContributor-Level 10
Thin layer chromatography is another type of adsorption chromatography silica gel of proper size spread over glass plate also acts as an adsorbent in thin layer chromatography size of 0.2 mm thick.
New answer posted
6 months agoContributor-Level 10
(i) Foam - Froth (e)
(ii) Gel - Jellies (c)
(iii) Aerosol - smoke (a)
(iv) Emulsion - milk (f)
New answer posted
6 months agoContributor-Level 10
Potential difference between fixed layer and mobile layer of lyophobic colloid is called zeta potential or electro kinetic potential.
This potential difference is related to charge on the surface of colloidal particles.
New answer posted
6 months agoContributor-Level 10
This is the method of preparation of lyophilic solution as condition is given.
New answer posted
6 months agoContributor-Level 10
For ideal gas using ;
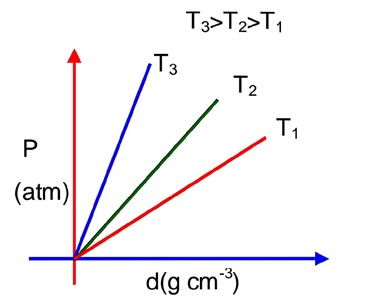
PM = dRT
Comparing with y = mx + C
Slope, m =
Slope
So; higher the slope higher the T
Hence, T3 > T2 > T1
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
