Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Get insights from 126 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Five, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
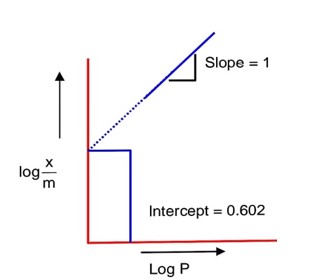
Using, Freundlich adsorption isotherm ;
………………. (i)
Comparing with y = mx + C
Slope =
Intercept, log k = 0.602
log k = log 4
k = 4
from equation (i)
= 0.12
= 12 * 10-2
So, 12 * 10-2 g of gas is adsorbed per gram of adsorbent,
New question posted
6 months agoNew answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Detergents with low CMC are more economical to use as they involve formation of micelle which is used for cleaning of oil and dirt from our cloth. Micelle formation takes place when the concentration of the detergent becomes equal to the CMC so the answer is (i).
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Greater the valence of the flocculating ion added, the greater is its power to cause precipitation. This is known as Hardy schulze rule. In the coagulation of a negative sol, the flocculating
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Colloidal particle shows Brownian movement. The Brownian movement has a stirring effect which does not permit the particles to settle and thus, is responsible for the stability of sols so the answer is (v).
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution. Hence, the values of colligative properties (osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point) are of small order as compared to values shown by true solutions at same concentration hence the answer is (i).
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Ans: Colloidal particles can pass through ordinary filter paper because the pores are too large. However, the pores of filter paper can be reduced in size by impregnating with collodion solution to stop the flow of colloidal particles hence the answer is (iii).
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: The answers are: (i)- (d) (ii)- (c) (iii)- (a) (iv)- (b)
Dispersed Phase | Dispersion medium | Example colloid solution |
Solid | Liquid | Butter |
Gas | Solid | Pumice stone |
Liquid | Liquid | Milk |
Solid | Liquid | Paint |
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i)- (d); (ii)- (c); (iii)- (a); (iv)- (b)
New answer posted
7 months agoContributor-Level 10
This is a Matching Type Questions as classified in NCERT Exemplar
Ans: (i) - (b); (ii) - (c); (iii) - (d); (iv) - (a)
S.No. | Dispersed Phase | Dispersion medium | Colloid |
(i) | Solid | Liquid | (b) Sol |
(ii) | Liquid | Solid | (c) Gel |
(iii) | Liquid | Liquid | (d) Emulsion |
(iv) | Gas | Liquid | (a) Foam |
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers