Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Get insights from 126 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Five, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Five
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: (ii) and (iv)
The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal
solution, because the repulsive forces between charged particles having same charge prevent them from coalescing or
aggregating when they come closer to one another. The Brownian movement has stirring effect which does not permit the particles to settle and thus, is responsible for the stability of sols hence the answer is (ii) and (iv).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: (ii) and (iii)
H2 molecule on an activated charcoal is adsorbed to a very little extent in comparison to easily liquefiable gases because it has (a) Very weak van there Waals force of attraction (b) Very low critical temperature hence the answer is (ii) and (iii).
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: (i) and (iii)
Freundlich gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbed and pressure at a particular temperature.
=kp
If = 0 ; = k xtent of adsorption is independent of pressure
When n=0 ; = kp
vs p is a line
parallel to x-axis
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: (i) and (ii)
The action of a catalyst is selective in nature and so a substance which acts as a catalyst in one reaction may fail to catalyse another reaction. They also do not change the enthalpy of reaction hence the answer is (i) and (ii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: (ii) and (iii)
The formation of micelles takes place only above a particular temperature which is the Kraft temperature (Tk ) and above a particular concentration i.e., the critical micelle concentration (CMC). Upon dilution, these colloids revert back to the individual ions hence the answer is (ii) and (iii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (ii)
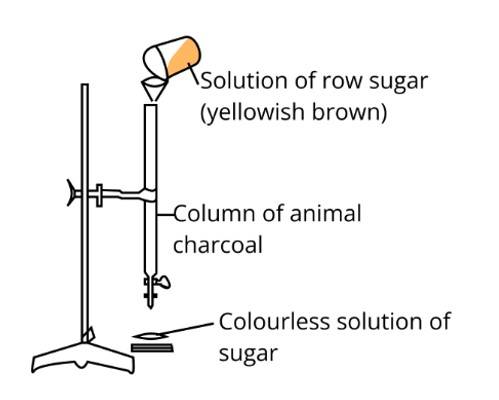
In the figure adsorption of coloured particle from charcoal is shown. Solution of raw sugar is filtered by animal charcoal
and yellowish brown colour of raw sugar is adsorbed and filterate is colourless which gives white colour on crystallization
hence the answer is (ii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct answer is (iv)
Charge on the sol particles can be a result of the following:
Due to electron capture by sol particles during electrodispersion of metals,
Due to preferential adsorption of ions from solution and/or
Due to formulation of electrical double layer.
Hence the correct answer is (iv)
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is iii.
River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in its deposition with the formation of delta hence the answeris (iii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct answer is (ii)
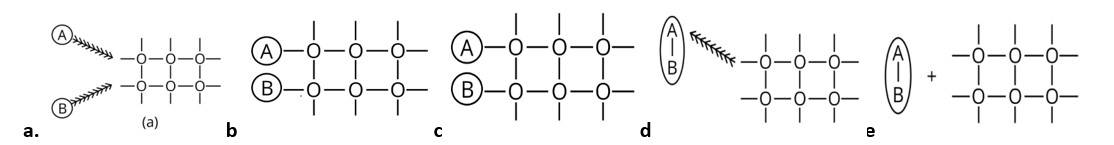
The correct sequence of steps involved in catalysis are:
(i) Adsorption of A and B on surface
(ii) Interaction between A and B to form intermediate
(iii) Starting of desorption from surface
(iv) Complete desorption from the surface
Therefore, the correct answer is (ii)
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers