Chemistry NCERT Exemplar Solutions Class 12th Chapter Six
Get insights from 105 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Six, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Six
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
32. Option (i)
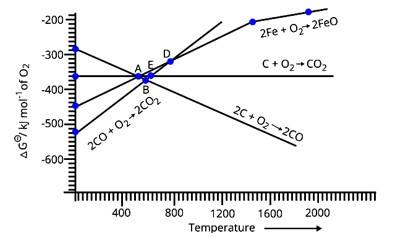
According to the above graph, at point D the equivalent value of ΔG for the reduction of FeO is approximately 330 units for the particular temperature. Also, for CO the equivalent value of Δ? at the particular temperature for point D is around −330 units. Thus, if we calculate the overall value of ΔG for the reduction of FeO with carbon monoxide then it is almost zero at point D.
New answer posted
7 months agoContributor-Level 10
31. Option (i)
Below point A, only the value of ΔG (CO, CO2) is less than the value of ΔG (Fe, FeO) at the corresponding temperatures. Thus, only carbon monoxide will be able to reduce FeO to Fe and will get itself oxidized into CO2.
New answer posted
7 months agoContributor-Level 10
29. Option (i)
The cyanide process involves 3 steps:
First step - The finely grounded ore of gold and silver are made to come in contact with the solution containing the cyanide,
Second step - it involves separation of gold and silver from the cyanide solution
Third step - it involves the recovery of gold and silver in their pure forms precipitating the remaining solution with zinc dust.
Thus, the metal is recovered by displacing Zn with the metal (Au or Ag) from metal ions.
New answer posted
7 months agoContributor-Level 10
28. Option (i)
The electrolytic method can be used to purify zinc and copper. The impure metal is used as an anode in this method. As the cathode, a pure strip of the same metal is used. They are immersed in an appropriate electrolytic bath containing a soluble salt of the same metal.
The more basic metals remain in the solution, while the less basic metals are transferred to the anode mud.
New answer posted
7 months agoContributor-Level 10
27. Option (ii)
Reaction involved in the metallurgy of aluminum is 2Al2O3 + 3C→ 4Al + 3CO2.
The reaction at cathode is Al3+ + 3e−→ Al
The reaction at anode is
C+12O2 →CO + 2e and C+O2→CO2 + 4e−
Hence, from the reaction graphite anode is oxidized to carbon monoxide and carbon dioxide.
New answer posted
7 months agoContributor-Level 10
26. Correct option (i)
The reaction at the anode with a lower E value is preferred, but oxygen cannot be obtained in this process due to overvoltage.
New answer posted
7 months agoContributor-Level 10
25. Option (iii)
Reaction takes place as Cu2O + Cu2S?3Cu +12SO2 with a product as bristle copper. This type of reaction is called an auto-reduction reaction as copper is reducing itself with help of other copper compounds.
New answer posted
7 months agoContributor-Level 10
24. Option (ii)
Purification of metal crystals by making a thin region of crystal undergo melting is known as Zone refining. The molten crystal is then moved up along the crystal to get pure form of it. This process is used to get pure form of Silicon and Germanium. Basic principle of the zone refining process is that the impurities are more soluble in molten metal than solid metal to get pure form of metal.
New answer posted
7 months agoContributor-Level 10
23. Option (i)
Explanation: Aluminum is the third most abundant metal in the earth's crust (8.3 percent approximately by weight). It is found in a variety of igneous minerals, including mica and clays. The second most abundant metal in the earth's crust is iron.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers