General Principles and Isolation of Elements
Get insights from 64 questions on General Principles and Isolation of Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about General Principles and Isolation of Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
is the temperature Co-efficient of cell. The cell having less variation of EMF, with respect to temperature have high efficiency.
New answer posted
6 months agoContributor-Level 10
Below 1350° C, Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO (from Ellingham diagram).
Melting and boiling point of Mg are lower than that of Al.
New answer posted
6 months agoContributor-Level 10
In ores/mineral available earthy and undesired impurities are gangue
New answer posted
6 months agoContributor-Level 10
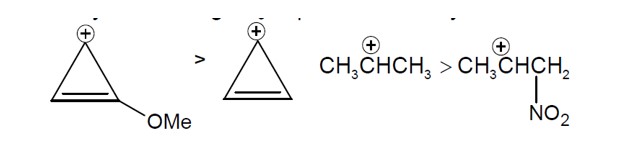
Sol. Reactivity towards depend on stability of carbocation formed
New answer posted
6 months agoContributor-Level 10
Leaching involves the given reaction,
Here, O2 is required for formation of Au (l) cyanide complex but no complex in absence of O2.
In above displacement reaction, Zn is oxidized.
New answer posted
6 months agoContributor-Level 10
Higher the E.N. difference between hydrogen and other atom then higher be the strength of intermolecular H-bond
Here, order of difference in E.N is
O - H > N – H > C - H
Hence, correct order of H bond strength is,
CH4 < HCN < NH3
New answer posted
7 months agoContributor-Level 10
55. (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: Hydrometallurgy is used to extract copper from low-grade ore. Hydrometallurgy entails dissolving the ore in a suitable reagent and then precipitating it. In this method, more electropositive metal is used, allowing pure metal to be displaced.
New answer posted
7 months agoContributor-Level 10
54. (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: The zone refining method is very useful for producing high-purity semiconductors and other metals, such as germanium.
New answer posted
7 months agoContributor-Level 10
53. (ii) Both assertion and reason are true but reason is not the correct explanation of assertion.
Explanation: Froth Flotation method is used to separate the hydrophobic materials from hydrophilic materials. In this method a mixture of palm oil, water and detergent is taken in a tank along with powdered sulfide ore. Compressed air is then passed through the pipe of the rotating agitator to create froth. The sulfide ore is then wetted by the palm oil mixture and it rises with the froth and the impurities or gauge settles at the bottom of the tank. The froth containing the sulfide is then cleaned and dried. Cresols or Aniline
New answer posted
7 months agoContributor-Level 10
52. (i) Both assertion and reason are true and reason is the correct explanation of assertion.
Explanation: Van Arkel method is generally used to obtain pure forms of Zirconium (Zr) and Titanium (Ti).
The metal iodide is heated at 1800 K and is decomposed on a tungsten filament. The pure metal is dropped on the tungsten filament. This proves that ZrI4 is volatile and decomposes at 1800 K.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers