Class 11th
Get insights from 8k questions on Class 11th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 11th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Option (ii)
Nodal plane is a plane which passes through the nucleus and the probability of finding an electron on a nodal plane is zero. Amongst the above given molecular orbital, only π*2px contains two nodal planes. Rest of the molecular orbitals, σ∗1, π2px, π*2py contains one nodal plane.
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: (i) The electronic configuration of dioxygen is:
σ1s2 σ∗1s2 σ2s2 σ∗2s2 s2pz2 π2p2y π2p2x π*2p1y π*2p1x
(ii) As it can be seen from the electronic configuration of oxygen atom π*2p1X π*2p1Y the are partially. So, the statement given is incorrect.
(iii) The statement given is correct because there are five bonding molecular orbitals and four antibonding molecular orbital in oxygen molecules. Hence, the bonding and antibonding molecular orbitals are no equal.
(iv) The filled bonding orbitals are not the same as the number of filled antibonding orbi
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
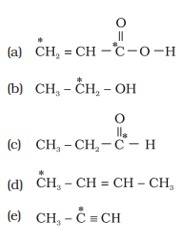
Ans: (i) Different types of hybridization in a carbon atom are:
(a) sp hybridization: The carbon atoms forming triple bonds with each other determines hybridized carbon. ( Cº C ).
(b) sp2 hybridization: The carbon atoms forming double bonds with each other determine sp2 hybridized carbon. (C=C). (c) sp3 hybridization: The carbon atoms forming single bonds with each other determine sp3 hybridized carbon. ( C-C).
(ii)The starred C atom is sp2 hybridised.
B] The starred C atom is sp3 hybridised.
C] The starred C atom is sp2 hybridised.
D] The starred C atom is sp3 hybridis
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Hybridisation in the case of PCl5 :
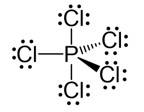
The hybridisation of phosphorus PCl5 is sp3d and it has a trigonal bipyramidal geometry. The axial bonds in PCl5 are slightly longer as compared to the equatorial bonds because axial bonds experience greater repulsive forces from other bonds as compared to the equatorial bonds.
Hybridisation in the case of SF6:
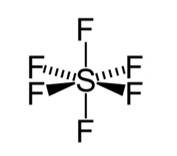
The hybridisation of sulphur in SF6 is sp3d2 and the molecule has an octahedral geometry. The bond length of axial as well as the equatorial bonds are similar because all the bonds in octahedral geometry experience similar r
New answer posted
7 months agoContributor-Level 10
This is a Long Answer Type Questions as classified in NCERT Exemplar
Ans: Formation of N2 molecule: Electronic Configuration
σ1s2 σ* < 1s2 < 2s2 < *2s2 < [ 2p2x = π2p2y] < 2p2z
The bond order will be:
( 10-4)= 3.0
Bond order indicates the number of bonds in a diatomic molecule. Triple bond N=N.
Formation of F2 molecule: Electronic Configuration
σ1s2 < *1s2< 2s2 < *2s2 < 2p2z < [ 2p2x = π2p2y] < [ *2p2x = π*2p2y] σ*2p2z
The bond order will be:
( 10-10)= 0
Hence there will be no bond formation in between the neon atoms.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (C) The combustion reaction of methane is given below
CH4 + O2 -> CO2 + H2O
In this reaction, water and carbon dioxide are formed.
According to the reaction, 1 mole of methane gives 2 moles of water. So, 16 g of methane gives 36 g of water
Thus, X is false but R is true
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (C)
In the decimal portion, only trailing or final zeros are significant. This is in accordance with the rules of significant figures.
The given number, 0.200 has 3 significant figures whereas the number, 200 has only one significant figure because zeros present on the right side or at the end of the decimal number are significant and it is provided that they are present on the right side of the decimal point.
Also, zeros are not significant in numbers without decimals. Thus, X is true but R is false.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (B)
Atomic mass unit is defined as a mass that is equal to accurately the mass of carbon-12 atom.
When scientists compare the relative atomic masses of the elements to the mass of carbon, they find that they are near to a whole number value.
So, both X and B are true but R is the correct explanation of A.
New answer posted
7 months agoContributor-Level 10
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(i) Both A and R are true and R is the correct explanation of A.
Explanation: The molecular formula gives the actual number of different atoms present in a molecule and the empirical formula gives a simple whole number ratio of different atoms present in a molecule.
Since ethene C2H4 can be further simplified, the empirical formula becomes CH2. The molecular mass of C2H4 is 28 g/mol and the empirical mass of CH2 is 14 g/mol.
Hence, the empirical mass of ethene is half of its molecular mass.
New answer posted
7 months agoContributor-Level 10
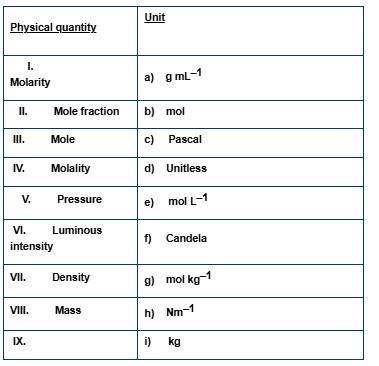
This is a Matching Type Questions as classified in NCERT Exemplar
(i) → (e) (ii) → (d) (iii) → (b) (iv) → (g) (v) → (c), (h) (vi) → (f) (vii) → (a) (viii) → (i)
Explanation:
(i) It is defined as the number of moles of solute dissolved in 1 litre of solution. So, the unit of molarity is mol L-1
(ii) Mole fraction is defined as the number of moles of a constituent divided by the total number of moles. So, it is unitless.
(iii) Mole is the unit to measure the amount of an atom or molecule in SI system. it is symbolized as "mol".
(iv) It is defined as the number of moles of solute dissolved in 1 kg of solvent, so, the unit o
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers