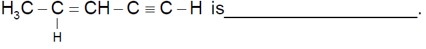- Some Basic Concepts of Chemistry Questions and Answers
- JEE Mains 2020
Some Basic Concepts of Chemistry Questions and Answers
| 1. A vessel contains 1.6 g of dioxygen at STP (273.15K, 1 atm pressure). The gas is now transferred to another vessel at constant temperature, where pressure becomes half of the original pressure. Calculate (i) volume of the new vessel. |
| Ans- P1=1 atm P2= 1/2=0.5 atm T1=273.15 K V2=? V1=? 32 g dioxygen occupies = 22.4 L volume at STP ∴ 1.6 g dioxygen will occupy = 22.4L x 1.6g / 32g = 1.12 L V1=1.12 L From Boyle's law (as temperature is constant) p1V1=p2V2 V2=p1V1p2 = 1 atm x 1.12 l/0.6 atm g = 2.24 L
(ii) number of molecules of dioxygen. Ans- (ii) Number of moles of dioxygen= Mass of dioxygen / Molar mass of dioxygen nO2 = 1.6/32 = 0.05 mol = 0.3011×1023 molecules =3.011×1022 molecules |
| 2. Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction given below: CaCO3 (s) + 2HCl (aq) → CaCl2(aq) + CO2(g) + H2O(l) What mass of CaCl2 will be formed when 250 mL of 0.76 M HCl reacts with 1000 g of CaCO3? Name the limiting reagent. Calculate the number of moles of CaCl2 formed in the reaction. |
| Ans: The volume of HCl solution is 250 mL and its molarity is 0.76M. The number of moles of HCl as follows, Moles of HCl Molarity Volume (in L) = 0.76M 0.250 L = 0.19 mol The molar mass of CaCO3 is 100 g / gQl and the mass of CaCO3 is given as 1000 g The number of moles of CaCO3 is calculated as Moles of CaCO3 = M a s s / M o l a r m a s s = 1000 g / 100 g / m o l = 10 mol According to the given reaction, 1 mole of CaCO3 requires 2 moles of HCl. So , the required number of moles of HCl for 10 moles of CaCO3 is calculated as Moles of HCl = 2mol of HCl /1mol of CaCO2 × 10 mol of CaCO3 = 20 mol So, the required number of moles of HCl is 20 mol but only 0.19 mol are given. So, HCl is a limiting reagent. So, the amount of calcium chloride formed is depend upon the limiting reagent, that is, the amount of HCl available. According to the reaction, 2 moles of HCl gives 1 mol of CaCl2 Sg 2 . , the number of moles of calcium chloride produced by 0.19 mol of HCl as follows, Moles of CaCl2 = 1 mol of CaCl2 / 2 mol of HCl x 0.19 mol of HCl = 0.095 mol The molar mass of calcium chloride is 111 g / mol. So, its mass is calculated as Mass of CaCl2 = Moles x Molar mass = 0.095 mol x 111 g / mol = 10.54 g |
| 3. Define the law of multiple proportions. Explain it with two examples. How does this law point to the existance of atoms? |
| Ans: According to the law of multiple proportions, when two elements react to form two or more than two chemical compounds, the ratio between different masses of one of the elements combining with a fixed mass of the other is always in the ratio of tiny numbers. Example: 1. Compounds of carbon and oxygen: C and O react to form two different compounds CO and CO2. In CO, 12 parts by mass of C reacts with 16 parts by mass of 0 . In CO2 ,12 parts by mass of C reacts with 32 parts by mass of O . If the mass of C is fixed at 12 parts of mass then the ratio in the masses of oxygen which reacts with the fixed mass of C is 16: 32, that is, 1: 2 . Therefore, the mass of oxygen contains a simple ratio of 1: 2 to each other. 2. Compounds of sulphur and oxygen: S and O reacts to form two compounds SO2 and SO3 . In SO2 ,32 parts by mass of S reacts with 32 parts by mass of O . In SO3 ,32 parts by mass of S reacts with 48 parts by mass of O . If the mass of S is fixed at 32 parts of mass then the ratio in the masses of oxygen which react with the fixed mass of S is 32: 48, that is, 2: 3. This law demonstrates that certain constituents combine in a specific proportion. It's possible that these constituents are atoms. As a result, the law of multiple proportions tells the existence of atoms that can join to form molecules. |
| 4. A box contains some identical red coloured balls, labelled as A, each weighing 2 grams. Another box contains identical blue coloured balls, labelled as B, each weighing 5 grams. Consider the combinations AB, AB2, A2B and A2B3 and show that law of multiple proportions is applicable. |
| Ans: In AB, 2 g of A combines with 5 g of B. So, 4 g of A combines with 10 g of B. In AB2 , 2g of B combines with 10 g of B, So 4g of A combines with 20 g of B. In A2B3 , 4G of B combines with 5 g of B. In A2B3 4 g of B combined with 15 g of B. So, the ratio between different masses of B which combine with fixed mass (4g) of A is 10: 20: 5: 15, that is, 2: 4: 1: 3. Hence, the ratio is simple. Therefore, the law of multiple proportions is applicable. |
Commonly asked questions
Define the law of multiple proportions. Explain it with two examples. How does this law point to the existance of atoms?
This is a Long Answer Type Questions as classified in NCERT Exemplar
According to the law of multiple proportions, when two elements react to form two or more than two chemical compounds, the ratio between different masses of one of the elements combining with a fixed mass of the other is always in the ratio of tiny numbers.
Example:
1. Compounds of carbon and oxygen:
C and O react to form two different compounds CO and CO2. In CO, 12 parts by mass of C reacts with 16 parts by mass of 0 .
In CO2 ,12 parts by mass of C reacts with 32 parts by mass of O .
If the mass of C is fixed at 12 parts of mass then the ratio in the masses of oxygen which reacts with the fixed mass of C is 16: 32, that is, 1: 2 .
Therefore, the mass of oxygen contains a simple ratio of 1: 2 to each other.
2. Compounds of sulphur and oxygen:
S and O reacts to form two compounds SO2 and SO3 .
In SO2 ,32 parts by mass of S reacts with 32 parts by mass of O . In SO3 ,32 parts by mass of S reacts with 48 parts by mass of O .
If the mass of S is fixed at 32 parts of mass then the ratio in the masses of oxygen which react with the fixed mass of S is 32: 48, that is, 2: 3.
This law demonstrates that certain constituents combine in a specific proportion. It's possible that these constituents are atoms. As a result, the law of multiple proportions tells the existence of atoms that can join to form molecules.
A vessel contains 1.6 g of dioxygen at STP (273.15K, 1 atm pressure). The gas is now transferred to another vessel at constant temperature, where pressure becomes half of the original pressure. Calculate
(i) Volume of the new vessel.
This is a Long Answer Type Questions as classified in NCERT Exemplar
P1=1 atm
P2= 1/2=0.5 atm
T1=273.15 K
V2=?
V1=?
32 g dioxygen occupies = 22.4 L volume at STP
∴ 1.6 g dioxygen will occupy = 22.4L x 1.6g / 32g = 1.12 L
V1=1.12 L
From Boyle's law (as temperature is constant)
p1V1=p2V2
V2=p1V1p2
= 1 atm x 1.12 l/0.6 atm g = 2.24 L
(ii) Number of molecules of dioxygen.
Ans- (ii) Number of moles of dioxygen= Mass of dioxygen / Molar mass of dioxygen
nO2 = 1.6/32 = 0.05 mol
1 mol of dioxygen contains = 6.022×1023 molecules of dioxygen
∴ 0.05 mol of dioxygen = 6.022×1023×0.05 molecule of O2
= 0.3011×1023 molecules
=3.011×1022 molecules
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction given below:
CaCO3 (s) + 2HCl (aq) → CaCl2(aq) + CO2(g) + H2O(l)
What mass of CaCl2 will be formed when 250 mL of 0.76 M HCl reacts with 1000 g of CaCO3? Name the limiting reagent. Calculate the number of moles of CaCl2 formed in the reaction.
This is a Long Answer Type Questions as classified in NCERT Exemplar
The volume of HCl solution is 250 mL and its molarity is 0.76M.
The number of moles of HCl as follows,
Moles of HCl Molarity Volume (in L)
= 0.76M 0.250 L
= 0.19 mol
The molar mass of CaCO3 is 100 g / gQl and the mass of CaCO3 is given as 1000 g
The number of moles of CaCO3 is calculated as
Moles of CaCO3 = M a s / M o l a r m a s
= 1000 g / 100 g / m o l = 10 mol
According to the given reaction, 1 mole of CaCO3 requires 2 moles of HCl. So, the required number of moles of HCl for 10 moles of CaCO3 is calculated as
Moles of HCl = 2mol of HCl /1mol of CaCO2 * 10 mol of CaCO3
= 20 mol
So, the required number of moles of HCl is 20 mol but only 0.19 mol are given. So, HCl is a limiting reagent. So, the amount of calcium chloride formed is depend upon the limiting reagent, that is, the amount of HCl available.
According to the reaction, 2 moles of HCl gives 1 mol of CaCl2 Sg 2 ., the number of moles of calcium chloride produced by 0.19 mol of HCl as follows,
Moles of CaCl2 = 1 mol of CaCl2 / 2 mol of HCl x 0.19 mol of HCl
= 0.095 mol
The molar mass of calcium chloride is 111 g / mol. So, its mass is calculated as
Mass of CaCl2 = Moles x Molar mass
= 0.095 mol x 111 g / mol
= 10.54 g
A box contains some identical red coloured balls, labelled as A, each weighing 2 grams. Another box contains identical blue coloured balls, labelled as B, each weighing 5 grams. Consider the combinations AB, AB2, A2B and A2B3 and show that law of multiple proportions is applicable.
This is a Long Answer Type Questions as classified in NCERT Exemplar
In AB, 2 g of A combines with 5 g of B.
So, 4 g of A combines with 10 g of B.
In AB2, 2g of B combines with 10 g of B, So 4g of A combines with 20 g of B.
In A2B3 , 4G of B combines with 5 g of B.
In A2B3 4 g of B combined with 15 g of B.
So, the ratio between different masses of B which combine with fixed mass (4g) of A is 10: 20: 5: 15, that is, 2: 4: 1: 3.
Hence, the ratio is simple. Therefore, the law of multiple proportions is applicable.
What will be the mass of one atom of C-12 in grams?
This is a Short Answer Type Questions as classified in NCERT Exemplar
Mass of 1 mole of C-12 = 12g
1 mole contains 6.022×1023 atoms.
Thus, mass of 6.022×1023 atoms=12g
Mass of 1 atom of carbon =126.022×1023 g
=1.99×10−23 g
Thus, mass of one atom of C-12 is 1.99×10−23 g
How many significant figures should be present in the answer of the following calculations?
This is a Short Answer Type Questions as classified in NCERT Exemplar
On solving the above equation, the result is 5.4. All non-zeroes digits are significant. The significant figure is 2.
What is the symbol for SI unit of mole? How is the mole defined?
This is a Short Answer Type Questions as classified in NCERT Exemplar
SI unit of the mole is mol. The amount of a substance that contains as many particles or entities as there are atoms in exactly
12 g (0.012 kg) of the C-12 isotope is defined as a mole. One mole is defined as follows:
1 mole = 6.023 x 1023
What is the difference between molality and molarity?
This is a Short Answer Type Questions as classified in NCERT Exemplar
The number of moles of a substance (known as the solute) dissolved in precisely 1 litce of a solution is known as molarity (solvent and solute combined). As a result, the formula for estimating molarity is as follows:
Molarity =
The term molarity is also used to refer to molar concentration. As a result, molar concentration measurement is based on the volume of liquid in which a substance is dissolved. It's vital to remember that the volume is in litres, so if we have volume in mL we need to convert that in liters.
Molality is the number of moles of substance (also known as the solute) found in a given mass of solvent (in Kg ) in which it is dissolved.
Molality is calculated by using the formula.
Major differences between Molarity and Molality are given as follows:
1) Molarity is the concentration of a material determined as the number of moles of solute dissolved in poe litre of solution, whereas molality is the concentration calculated as the number of moles of solute found in one kilogram of solvent.
2) Molality is denoted by the symbol m, whereas molarity is denoted by the symbol M.
3) The molarity formula is moles per litre, but the molality formula is moles per kilogram.
4) Molarity is impacted by temperature changes, whereas molality is unaffected by temperature changes.
5) Changes in pressure affect molarity, but they do not affect molality.
6) Molarity can lead to an imprecise and inaccurate concentration, whereas molality can lead to an accurate and precise concentration measurement.
Calculate the mass percent of calcium, phosphorus and oxygen in calcium phosphate Ca3(PO4)2.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Molecular mass of Ca3 (PO4)2 = 310.18 g / mole
Given mass of calcium 4 x 30= 120 g
Given mass of phosphorous = 31 x 2 =62 g
Given mass of oxygen = 16 x 8 = 128 g
Mass percent of Calcium = x 100 = 38.71%
Mass percent of Phosphorous = x 100 = 20%
Mass percent of Oxygen = x 100 = 41.29 %
45.4 L of dinitrogen reacted with 22.7 L of dioxygen and 45.4 L of nitrous oxide was formed. The reaction is given below:
2N2(g) + O2(g) → 2N2O(g)
Which law is being obeyed in this experiment? Write the statement of the law?
This is a Short Answer Type Questions as classified in NCERT Exemplar
If all gases are at the same temperature and pressure, Gay lussac's law of gaseous volumes states that gases combine or are created in a chemical reaction in a simple volume ratio.
H2 (g) + Cl2 (g) → 2HCL (g)
1 volume 1 volume 2 volume
22.4 litre 22.4 litre 44.8 litre
2N2 (g) + O2 (g) → 2N2O (g)
2Vol 1Vol 2Vol
45.4 litre 22.7 litre 45.4 litre
2: 1: 2
The above examples proved that gaseous volumes combine in a simple volume ratio.
If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in whole number ratio.
This is a Short Answer Type Questions as classified in NCERT Exemplar
(a) Is this statement true?
Ans: Yes the given statement is true
(b) If yes, according to which law?
Ans: Multiple law of proportions: According to the Law of Multiple proportions, when two elements combine to generate more than one compound, the weights of one element that combine with a fixed weight of the other are in a ratio of tiny whole numbers.
(c) Give one example related to this law
Ans:
C (g) + O (g) -> CO (g)
12 g 16 g 28 g
C (g) + O2 (g) -> CO2 (g)
12 g 32 g 44 g
This mass of Oxygen, which combines with a fixed mass of Carbon is in the simple ratio that is 16:32 or 1:2.
Calculate the average atomic mass of hydrogen using the following data :
|
Isotope |
% Natural abundance |
Molar mass |
|
1H |
99.985 |
1 |
|
2H |
0.015 |
2 |
This is a Short Answer Type Questions as classified in NCERT Exemplar
(Natural abundance of 1H x molar mass ) + (Natural abundance of 2H x molar mass of 2H)
Natural abundance of 1H = 99.985
Natural abundance of 2H = 0.015
Average atomic mass =
= 1.00015u
Hydrogen gas is prepared in the laboratory by reacting dilute HCl with granulated zinc. Following reaction takes place.
Zn + 2HCl → ZnCl2 + H2
Calculate the volume of hydrogen gas liberated at STP when 32.65 g of zinc reacts with HCl. 1 mol of a gas occupies 22.7 L volume at STP; atomic mass of Zn = 65.3 u.
This is a Short Answer Type Questions as classified in NCERT Exemplar
65.3 g of Zinc gives 22.7 litres of Hydrogen gas
32.65 g Zinc gives = 32.65g x 22.7 litres/65.3 = 11.35 L
The density of 3 molal solution of NaOH is 1.110 g mL–1. Calculate the molarity of the solution.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Mass of NaoH = 40 g
Mass of solvent =1000 g
Mass of solution = 40 x 3+1000
Density =
=
= = 1009.0 mL
Molarity = = 2.97M
1009.00 mL= 1.009 L
Hence, the molarity of the solution is 2.97M
Volume of a solution changes with change in temperature, then, will the molality of the solution be affected by temperature? Give reason for your answer.
This is a Short Answer Type Questions as classified in NCERT Exemplar
Molality is the number of moles of substance (also known as the solute) found in a given mass of solvent (in Kg ) in which it is dissolved. Molality is calculated by using the formula.
Molality =
So, temperature has no effect on the molality of the solution because molality is expressed in mass.
If 4 g of NaOH dissolves in 36 g of H2O, calculate the mole fraction of each component in the solution. Also, determine the molarity of solution (specific gravity of solution is 1g mL–1).
This is a Short Answer Type Questions as classified in NCERT Exemplar
Molar mass of NaOH = 40 g/ mole
Molar mass of water =18 g / mole
Mass of NaOH= 4 g
Number of moles of 4 g NaOH = = 0.1 mol
Number of moles of H2O= =2 mol
Mole fraction of water
Mole fraction of NaOH = = = 0.95
Number of moles of= = = 0.047
Number of moles of NaOH
Mass of solution = Mass of solute + Mass of solvent
Mass of NaOH + Mass of water 4 g + 36 g = 40 g
Specific gravity of solution =1 g / ml
1 litre =1000 ml volume of solution = 40ml
40ml = 0.04 litre
Molarity = = = 2.5M
The reactant which is entirely consumed in reaction is known as limiting reagent. In the reaction
2A + 4B → 3C + 4D, when 5 moles of A react with 6 moles of B, then
(i) Which is the limiting reagent?
This is a Short Answer Type Questions as classified in NCERT Exemplar
The reaction is shown below.
2A + 4B? 3C + 4D
According to the above equation, 2 moles of A requires 4 moles of B. So, the number of moles of B required for 5 moles of A is calculated as,
Moles of B = 5 mol of A * = 10 mol of B
So, the required number of moles of B is 10 mal but only 6 moles of B are given in the question. Therefore, B is the limiting reagent.
(ii) calculate the amount of C formed?
Ans: Now, the amount of C can be calculated by the limiting reagent, that is, the amount of B.
According to the equation, 4 moles of B gives 3 moles of C . So, the number of moles of C formed by 6 moles of B as follows,
Moles of C = 6 mol of B * = 4.5 mol of C
Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below. Correct reading of mass is 3.0 g. On the basis of given data, mark the correct option out of the following statements.
Student Readings
(i) (ii)
A 3.01 2.99
B 3.05 2.95
(i) Results of both the students are neither accurate nor precise.
(ii) Results of student A are both precise and accurate.
(iii) Results of student B are neither precise nor accurate.
(iv) Results of student B are both precise and accurate.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (ii)
Average of readings of student A,
Average of student A =
Average of readings of student B,
Average of student B =
The correct reading is 3. For both A and B, the average value is close to the correct value. Thus, readings of both are accurate.
The readings of student A are also very close to each other and close to the average value. Thus, readings are precise. Also, the readings of student B are also very close to each other and close to the average value.
A measured temperature on Fahrenheit scale is 200 ° F. What will this reading be on a Celsius scale?
(i) 40 °C
(ii) 94 °C
(iii) 3 °C
(iv) 30 °C
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
For the conversion from Fahrenheit to Celsius
On substituting the values in the above equation,
200 =
=93.3 C
What will be the molarity of a solution, which contains 5.85 g of NaCl(s) per 500 mL?
(i) 4 mol L–1
(ii) 20 mol L–1
(iii) 0.2 mol L–1
(iv) 2 mol L–1
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C)
No. of mole given =
On substituting the value in the above equation, the cal, can be calculated as
no, of mole = = 0.1 g
The molarity (M) is given by the formula:
M =
On substituting the values in the above equation:
Molarity =
= 0.2 mol L-1
If 500 mL of a 5M solution is diluted to 1500 mL, what will be the molarity of the solution obtained?
(i) 1.5 M
(ii) 1.66 M
(iii) 0.017 M
(iv) 1.59 M
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
The relation between molarity and volume is given as,
M1 V1= M2 V2
On substituting the value in the above equation, the political can be calculated as
5M* 500 mL = M2*1500 mL
M =1.66M
The number of atoms present in one mole of an element is equal to Avogadro number. Which of the following element contains the greatest number of atoms?
(i) 4g He
(ii) 46g Na
(iii) 0.40g Ca
(iv) 12g He
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (D)
(A) The number of moles is given by the following formula,
Moles = ….(1)
The number of moles of He is calculated by using equation (1) as follows
Moles of O2 = =1 mol
The number of atoms can be calculated as, number of moles
….(2)
On substituting the values in the above equation:
1 mol =
Number of atoms = 1 6.022 1023
(B) The number of moles of Na is calculated by using equation (1) as follows,
Moles of Na = = 2 mol
The number of atoms can be calculated by using equation (2) as follows,
2 mol =
number of atoms= 2 6.022 1023
(C) The number of moles of Ca is calculated by using equation (1) as follows
Moles of Ca= = 0.01 mol
The number of atoms can be calculated by using equation (2) as follow
0.01 mol =
number of atoms = 0.01 6.022 1023
(D) The number of moles of He is calculated by using equation (1) as follows,
Moles of He = = 3 mol
The number of atoms can be calculated by using equation (2) as follows,
3 mol=
number of atoms = 3 6.022 1023
Thus, 12 g He contains the highest number of atoms.
If the concentration of glucose (C6H12O6) in blood is 0.9 g L–1, what will be the molarity of glucose in blood?
(i) 5 M
(ii) 50 M
(iii) 0.005 M
(iv) 0.5 M
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C)
The molar mass of glucose is 180 g mol- . The molarity (M) is given by the formula:
M =
On substituting the value in the above equation, the mol can be calculated as
M = = 0.005M
What will be the molality of the solution containing 18.25 g of HCl gas in 500 g of water?
(i) 0.1 m
(ii) 1 M
(iii) 0.5 m
(iv) 1 m
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (D)
The number of moles is given by the following formula,
Moles = …. (1)
The number of moles of HCl is calculated by using equation (1) as follows,
Moles of HCl 0.5 mol
The molality (m) is given by the formula:
m =
On substituting the values in the above equation:
Molality = = 1m
One mole of any substance contains 6.022 × 1023 atoms/molecules. Number of molecules of H2SO4 present in 100 mL of 0.02M H2SO4 solution is ______
(i) 12.044 × 1020 molecules
(ii) 6.022 × 1023 molecules
(iii) 1 × 1023 molecules
(iv) 12.044 × 1023 molecules
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A)
The molarity (M) is given by the formula:
M
On substituting the values in the above equation:
0.02M =
n = 0.002 mol
The number of molecules can be calculated as, number of moles
=
On substituting the values in the above equation:
0.002 mol =
number of molecules = 0.002 6.022 1023
= 12.044 1020
What is the mass percent of carbon in carbon dioxide?
(i) 0.034%
(ii) 27.27%
(iii) 3.4%
(iv) 28.7%
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
The molar mass of carbon is 44 g mol-1
44 g of carbon dioxide contains 12 g of carbon
The percentage composition is given as, % composition
=
On substituting the values in the above equation,
% of carbon =
= 27.27%
The empirical formula and molecular mass of a compound are CH2O and 180 g respectively. What will be the molecular formula of the compound?
(i) C9H18O9
(ii) CH2O
(iii) C6 H12O6
(iv) C2H4O2]
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C)
The empirical formula mass of CH2O is 30 g
The relation between empirical and molecular formula is given as,
n = …. (1)
On substituting the values in equation (1)
n = = 6
Thus, the molecular formula of the compound will be,
Molecular formula of the compound = (CH2O)6 = C6H12O6
If the density of a solution is 3.12 g mL–1, the mass of 1.5 mL solution in significant figures is _______.
(i) 4.7g
(ii) 4680 × 10–3g
(iii) 4.680g
(iv) 46.80g
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A)
The density (d ) is given by the formula:
d =
Here, m is the mass and v is the volume
On substituting the values in the above equation:
3.12 g mL-1 = M = 4.68 g
The mass will be 4.7 upto 2 significant figure.
Which of the following statements about a compound is incorrect?
(i) A molecule of a compound has atoms of different elements.
(ii) A compound cannot be separated into its constituent elements by physical methods of separation.
(iii) A compound retains the physical properties of its constituent elements.
(iv) The ratio of atoms of different elements in a compound is fixed.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C)
(A) The molecule of a compound is obtained when two or more atoms of different elements are combined together.
(B) A compound can be separated into its constituent elements by physical methods of separation.
(C) A compound does not retains the physical and chemical properties of its constituent elements.
(D) When two elements react to form two or more than two chemical compounds, the ratio between different masses of one of the elements combining with a fixed mass of the other.
Which of the following statements is correct about the reaction given below:
4Fe(s) + 3O2(g) → 2Fe2O3(g)
(i) Total mass of iron and oxygen in reactants = total mass of iron and oxygen in product therefore it follows law of conservation of mass.
(ii) Total mass of reactants = total mass of product; therefore, law of multiple proportions is followed.
(iii) Amount of Fe2O3 can be increased by taking any one of the reactants (iron or oxygen) in excess.
(iv) Amount of Fe2O3 produced will decrease if the amount of any one of the reactants (iron or oxygen) is taken in excess.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A)
According to the law of conservation of mass, matter can neither be created nor be destroyed. In the given reaction,
4Fe + 3O2→ 2Fe2O3
The mass of reactant is equal to the mass of product. Hence, it follows the law of conservation of mass
Which of the following reactions is not correct according to the law of conservation of mass
(i) 2Mg(s) + O2(g) → 2MgO(s)
(ii) C3H8(g) + O2(g) → CO2(g) + H2O(g)
(iii) P4(s) + 5O2(g) → P4O10(s)
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O (g)
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
In a, c, and d options given reactions, all the elements are balanced and hence, following the law of conservation of mass.
In reaction (B), C3H8+ O2→ CO2+H20 elements are not balanced. Hence product are different. It is against the law of conservation of mass
Which of the following statements indicates that law of multiple proportion is being followed.
(i) Sample of carbon dioxide taken from any source will always have carbon and oxygen in the ratio 1:2.
(ii) Carbon forms two oxides namely CO2 and CO, where masses of oxygen which combine with fixed mass of carbon are in the simple ratio 2:1.
(iii) When magnesium burns in oxygen, the amount of magnesium taken for the reaction is equal to the amount of magnesium in magnesium oxide formed.
(iv) At constant temperature and pressure 200 mL of hydrogen will combine with 100 mL oxygen to produce 200 mL of water vapour.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
Law of multiple proportion: According to the law of multiple proportion, whe form two or more than two chemical compounds, the ratio between different elements combining with a fixed mass of the other is always in the ratio of tin Compounds of carbon and oxygen:
Carbon and oxygen react to form two different compounds CO and CO2. In Carbon react with 16 parts by mass of oxygen.
In CO2 ,12 parts by mass of Carbon react with 32 parts by mass of oxygen. If the mass of Carbon is fixed at 12 parts of mass then the ratio in the masses with the fixed mass of carbon is 16: 32, that is, 1: 2. Therefore, the mass of oxygen contains a simple ratio of 2: 1.to each other
In the following questions two or more options may be correct.
One mole of oxygen gas at STP is equal to _______.
(i) 6.022 × 1023 molecules of oxygen
(ii) 6.022 × 1023 atoms of oxygen
(iii) 16 g of oxygen
(iv) 32 g of oxygen
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A), (D)
One mole of oxygen gas at STP is equal to 6.022 x 1023 molecules of oxygen The number of moles is given by the following formula,
Moles = ----------(1)
The number of moles of O2 is calculated by using equation (1) as follow.
Moles of O2 =
= 0.5 mol
The number of molecules can be calculated as, number of moles
= ----------(2)
On substituting the values in the above equation:
0.5 mol =
number of molecules = 0.5 6.022 1023
The number of moles is given by the following formula,
moles = ----------(1)
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 =
=1 mol
The number of molecules can be calculated as, number of moles
= ----------(2)
On substituting the values in the above equation:
1 mol =
Number of molecules = 1 6.022 1023
Sulphuric acid reacts with sodium hydroxide as follows :
H2SO4 + 2NaOH → Na2SO4 + 2H2O
When 1L of 0.1M sulphuric acid solution is allowed to react with 1L of 0.1M sodium hydroxide solution, the amount of sodium sulphate formed and its molarity in the solution obtained is
(i) 1 mol L–1
(ii) 10 g
(iii) 025 mol L–1
(iv) 55 g
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B), (C)
0.1 mole H2SO4 reacts with 1 mole of NaOH.
0.1 mole of NaOH will react with = mole of H2SO4
Here, NaOH is the limiting reagent.
2 mole of NaOH produces 1 mole of Na2SO4
0.1 mole of NaOH will give mole of Na2SO4
No. of mole =
On substituting the value in the above equation, the mass can be calculated as
0.05 mol =
given mass = 7.10 g
Volume of solution after mixing is 2 L.
So, the molarity of Na2SO4 is
Molarity = = 0.025mol L-1
Which of the following pairs have the same number of atoms?
(A) 16g of O2 and 4g of H2
(B) 16g of O2 and 44g of CO2
(c) 28g of N2 and 32g of O2
(D) 12g of c and 23g of Na
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option(C), (D)
(A) The number of moles is given by the following formula,
Moles = -----------(1)
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 = = 0.5 mol
The number of atoms can be calculated as, number of moles
-----------(2)
On substituting the values in the equation (2)
0.5 mol =
number of atoms = 0.5 6.022 1023
The number of moles of H2 is calculated by using equation (1) as follows,
Moles of H2 =
= 2 mol
On substituting the values in the equation (2):
2 mol =
number of atoms = 2 6.022 1023
(B) The number of moles of CO2 is calculated by using equation (1) as follows
Moles of CO2 =
= 1 mol
On substituting the values in the equation (2), the number of atoms can be calculated as ,
1 mol =
number of atoms = 1 6.022 1023
(C) The number of moles of N2 is calculated by using equation (1) as follows,
Moles of N2 = =1 mol
On substituting the values in the equation (2), the number of molecules can be calculated as
1 mol =
number of atoms = 1 6.022 1023
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 = = 1
The number of atoms can be calculated as, number of moles
-
On substituting the values in the equation (2)
1 mol =
number of atoms = 1 6.022 1023
(D) The number of moles of C is calculated by using equation (1) as follows,
Moles of C = =1 mol
On substituting the values in the equation (2), the number of molecules can be calculated as
1 mol =
number of atoms = 1 6.022 1023
The number of moles of Na is calculated by using equation (1) as fo
Moles of Na = =1 mol
On substituting the values in the equation (2), the number of molecules can be calculated as
1 mol =
number of atoms = 1 6.022 1023
Which of the following solutions have the same concentration?
(i) 20 g of NaOH in 200 mL of solution
(ii) 5 mol of KCl in 200 mL of solution
(iii) 40 g of NaOH in 100 mL of solution
(iv) 20 g of KOH in 200 mL of solution
This is a Multiple Choice Questions as classified in NCERT Exemplar
The answers are options (i) and (ii)
(A)The number of moles is given by the following formula,
Moles =
So, the number of moles of NaOH is calculated, by using equation (1) as follows
20 g NaOH in 200 mL solution.
Moles of NaOH= mol of NaOH
The molarity (M ) is given by the formula:
M=
On substituting the values in the above equation:
M (NaOH) =
= 2.5 mol L-1
(B) The molarity is given by the formula:
M=
On substituting the values in the above equation:
M (KCL) =
= 2.5 mol L-1
16 g of oxygen has same number of molecules as in
(i) 16 g of CO
(ii) 28 g of N2
(iii) 14 g of N2
(iv) 1.0 g of H2
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option(C), (D)
The number of moles is given by the following formula,
Moles = ---------------(1)
The number of molecules can be calculated as, number of moles
= ----------(2)
(C) The number of moles of N2 is calculated by using equation (1) as follows,
Moles of N2 = = 0.5 mol
The number of molecules can be calculated by using equation (2) as follows,
0.5 mol =
number of molecules = 0.5 6.022 1023
(D) The number of moles of H2 is calculated by using equation (1) as follows,
Moles of H2 = = 0.5 mol
The number of molecules can be calculated by using equation (2) as follows,
0.5 mol =
number of molecules = 0.5 6.022 1023
Which of the following terms are unitless?
(i) Molality
(ii) Molarity
(iii) Mole fraction
(iv) Mass percent
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (C), (D)
Molality: It is defined as the number of moles of solute divided by the mass of solvent in kg . It is denoted by m . The formula is expressed as
m =
The SI unit of molality is 1 mol/kg
Molarity: It is defined as the number of moles of solute divided by the volume of solution in latice. It is represented by M The formula of molarity is expressed as
M =
Here, n is the number of moles of solute and V is the volume of solution expressed in liters. The SI unit of molarity is mol/ L .
Mole fraction (x ) : The mole fraction of a substance is defined as the number of moles of that substance divided by the total number of moles of all substances present in the solution.
Mass percent: it is defined as the weight of the solute divided by the total weight of the solution and the obtained result is multiplied by 100 in order to give a percent. Thus, mole fraction and mass percent are unitless.
One of the statements of Dalton’s atomic theory is given below: “Compounds are formed when atoms of different elements combine in a fixed ratio” Which of the following laws is not related to this statement?
(i) Law of conservation of mass
(ii) Law of definite proportions
(iii) Law of multiple proportions
(iv) Avogadro law
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (A), (D)
Law of conservation of mass: According to this law, the mass is neither created nor destroyed during a chemical reaction in an isolated system. It can only be transformed from one form to another. Burning of wood is an example of conservation of mass. Law of definite proportion:
According to this law, a compound contains exactly the same elements in the fixed proportion by mass. For example, pure water consists of 11.196 H and 88.9% o by mass.
Law of multiple proportion: According to the law of multiple proportion, when two elements react to form two or more than two chemical compounds, the ratio between different masses of one of the elements combining with a fixed mass of the other is always in the ratio of tiny numbers. For example, compounds of carbon and oxygen. Avogadro's law: This law states that the number of moles of a gas is directly related to the volume enclosed by the gas at constant pressure and temperature.
Match the following:
|
Column I |
Column II |
|
(i) 88 g of CO2 |
(a) 0.25 mol |
|
(ii) 6.022×1023 molecules of H2O |
(b) 2 mol |
|
(iii) 5.6 litres of O2 at STP |
(c) 1 mol |
|
(iv) 96 g of O2 |
(d) 6.022×1023 molecules |
|
(v) 1 mol of any gas |
(e) 3 mol |
This is a Matching Type Questions as classified in NCERT Exemplar
(i)- (b); (ii) - (c); (iii) - (a); (iv) - (e); (v) - (d)
(i) 88 g of CO2
The number of moles is given by the following formula,
Moles = - (1)
So, the number of moles of CO2 is calculated by using equation (1) as follows
Moles of CO2 = = 2 mol
Thus, option (i) from column I is matched with (b) from column II
(ii) 1 mol of H2O gives 6.022*1023 molecules. So, 6.022*1023 molecules contain 1 mol of H2O . Thus, option (ii) from column I is matched with (b) from column II
(iii) 5.6 liters of O2 at STP
1 mol of gas occupies 22.4 liters of O2 . For 5.6 liters of O2, the number of moles of oxygen gas is calculated as
Moles of O2 = * 5.6 L = 0.25 mol
(iv) 96 g of O2
The number of moles of O2 is calculated by using equation (1) as follows,
Moles of O2 = = 3 mol
Thus, option (iv) from column I is matched with (e) from column II.
(v) 1 mol of any gas 1 mole of any gas contains 6.022*1023 molecules.
Thus, option (v) from column I is matched with (d) from column II.
Match the following physical quantities with units.
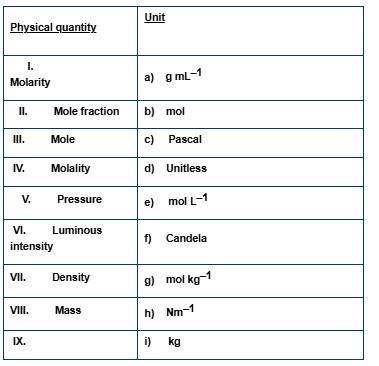
This is a Matching Type Questions as classified in NCERT Exemplar
(i) → (e) (ii) → (d) (iii) → (b) (iv) → (g) (v) → (c), (h) (vi) → (f) (vii) → (a) (viii) → (i)
Explanation:
(i) It is defined as the number of moles of solute dissolved in 1 litre of solution. So, the unit of molarity is mol L-1
(ii) Mole fraction is defined as the number of moles of a constituent divided by the total number of moles. So, it is unitless.
(iii) Mole is the unit to measure the amount of an atom or molecule in SI system. it is symbolized as "mol".
(iv) It is defined as the number of moles of solute dissolved in 1 kg of solvent, so, the unit of morality is mol kg-1.
(v) It is defined as the force per unit area. so, the unit of pressure is Pascal.
(vi) It is the amount of visible light released per unit solid angle in unit time. so, it's unit will be Candela.
(vii) It is defined as the mass of a substance (in kg) divided by the volume of that substance (in mL). The unit of density is g ml-1
(viii) Mass is used to measure the amount of a substance or an object. The unit of mass is kg.
In the following questions a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the choices given below each question.
Assertion (A): The empirical mass of ethene is half of its molecular mass.
Reason (R): The empirical formula represents the simplest whole number ratio of various atoms present in a compound.
(i) Both A and R are true and R is the correct explanation of A.
(ii) A is true but R is false.
(iii) A is false but R is true.
(iv) Both A and R are false
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
(i) Both A and R are true and R is the correct explanation of A.
Explanation: The molecular formula gives the actual number of different atoms present in a molecule and the empirical formula gives a simple whole number ratio of different atoms present in a molecule.
Since ethene C2H4 can be further simplified, the empirical formula becomes CH2. The molecular mass of C2H4 is 28 g/mol and the empirical mass of CH2 is 14 g/mol.
Hence, the empirical mass of ethene is half of its molecular mass.
Assertion (A) : One atomic mass unit is defined as one twelfth of the mass of one carbon-12 atom.
Reason (R) : Carbon-12 isotope is the most abundunt isotope of carbon and has been chosen as standard.
(i) Both A and R are true and R is the correct explanation of A.
(ii) Both A and R are true but R is not the correct explanation of A.
(iii) A is true but R is false.
(iv) Both A and R are false.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (B)
Atomic mass unit is defined as a mass that is equal to accurately the mass of carbon-12 atom.
When scientists compare the relative atomic masses of the elements to the mass of carbon, they find that they are near to a whole number value.
So, both X and B are true but R is the correct explanation of A.
Assertion (A) : Significant figures for 0.200 is 3 where as for 200 it is 1.
Reason (R) : Zero at the end or right of a number are significant provided they are not on the right side of the decimal point.
(i) Both A and R are true and R is correct explanation of A.
(ii) Both A and R are true but R is not a correct explanation of A.
(iii) A is true but R is false.
(iv) Both A and R are false.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (C)
In the decimal portion, only trailing or final zeros are significant. This is in accordance with the rules of significant figures.
The given number, 0.200 has 3 significant figures whereas the number, 200 has only one significant figure because zeros present on the right side or at the end of the decimal number are significant and it is provided that they are present on the right side of the decimal point.
Also, zeros are not significant in numbers without decimals. Thus, X is true but R is false.
Assertion (A) : Combustion of 16 g of methane gives 18 g of water.
Reason (R) : In the combustion of methane, water is one of the products.
(i) Both A and R are true but R is not the correct explanation of A.
(ii) A is true but R is false.
(iii) A is false but R is true.
(iv) Both A and R are false.
This is a Assertion and Reason Type Questions as classified in NCERT Exemplar
Option (C) The combustion reaction of methane is given below
CH4 + O2 -> CO2 + H2O
In this reaction, water and carbon dioxide are formed.
According to the reaction, 1 mole of methane gives 2 moles of water. So, 16 g of methane gives 36 g of water
Thus, X is false but R is true
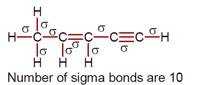
The number of sigma bonds in
Number of sigma bonds are 10.
[Structure showing 10 sigma bonds in the molecule]
At 298 K, the enthalpy of fusion of a solid (X) is 2.8kJ mol?¹ and the enthalpy of vaporization of the liquid (X) is 98.2 kJ mol?¹. The enthalpy of sublimation of the substance (X) in kJ mol?¹ is ______ (in nearest integer).
ΔH? = ΔH? + ΔH?
= 2.8 + 98.2 = 101 kJ/mole
Consider the complete combustion of butane, the amount of butane utilized to produce 72.0 g of water is ______ × 10?¹g, (in nearest integer).
C? H? + 13/2 O? → 4CO? + 5H? O
1 mole C? H? (58 g) produces 5 mole H? O (90 g)
∴ 90 g H? O obtained from 58 g C? H?
∴ 72g H? O obtained from (58/90) × 72g = 46.4 g
= 464 × 10? ¹g
CO? gas is bubbled through water during a soft drink manufacturing process at 298 K. If CO? exerts a partial pressure of 0.835 bar then x m mol of CO? would dissolve in 0.9 L of water. The value of x is ______ (Nearest integer). (Henry's law constant for CO? at 298 K is 1.67×10³ bar)
P (CO? ) = K? X (CO? )
X (CO? ) = P (CO? )/K? = 0.835 / (1.67 × 10³) = 0.5 × 10? ³
X (CO? ) = n (CO? )/ (n (CO? ) + n (H? O) ≈ n (CO? )/n (H? O) (since n (CO? ) << n (H? O)
n (H? O) in 0.9L = 900g/18gmol? ¹ = 50 mol
n (CO? ) = X (CO? ) × n (H? O) = 0.5 × 10? ³ × 50 = 25 × 10? ³ moles = 25 mmol
When 10 ml of an aqueous solution of Fe²? ions was titrated in the presence of dil H?SO? using diphenylamine indicator, 15 mL of 0.02 M solution of K?Cr?O? was required to get the end point. The molarity of the solution containing Fe²? ions is x × 10?²M. The value of x is ______. (Nearest integer)
Cr? O? ²? + 6Fe²? + 14H? → 2Cr³? + 6Fe³? + 7H? O
n-factor for Cr? O? ²? = 6, for Fe²? = 1
M.E Cr? O? ²? = M.E Fe²?
M? V? n? = M? V? n?
0.02 × 15 × 6 = M? × 10 × 1
M? = (0.02 × 15 × 6)/10 = 0.18 M = 18 × 10? ² M
Consider the cell at 25°C
Zn | Zn²?(aq), (1M) || Fe³?(aq), Fe²?(aq) | Pt(s)
The fraction of total iron present as Fe³? ion at the cell potential of 1.500V is x × 10?². The value of x is ______ (Nearest integer).
(Given: E?(Fe³?/Fe²?) = 0.77V, E?(Zn²?/Zn) = -0.76V)
Fe? ³ + e? → Fe? ² E° = 0.77V
Zn (s) → Zn? ² + 2e? ; E° = 0.76V
Cell reaction: 2Fe? ³ + Zn → 2Fe? ² + Zn? ² E°cell = 1.53V
Ecell = E°cell - (0.059/2)log ( [Zn? ²] [Fe? ²]²/ [Fe? ³]²)
1.5 = 1.53 - (0.06/2)log (1 × [Fe? ²]²/ [Fe? ³]²)
-0.03 = -0.03 log ( [Fe? ²]/ [Fe? ³])²
1 = log ( [Fe? ²]/ [Fe? ³])² => [Fe? ²]/ [Fe? ³] = 10
Let total iron = T. [Fe? ³] + [Fe? ²] = T. [Fe? ³] + 10 [Fe? ³] = T. 11 [Fe? ³] = T.
fraction of Fe? ³ = [Fe? ³]/T = 1/11 ≈ 0.09
This solution seems to differ from the image. Let's follow the image's steps.
log ( [Fe? ²]/ [Fe? ³])² = 1 => ( [Fe? ²]/ [Fe? ³])² = 10
[Fe? ²]/ [Fe? ³] = √10 = 3.16. Then [Fe? ³]/ [Fe? ²] = 1/√10 = 0.316.
fraction of [Fe? ³] = [Fe? ³]/ ( [Fe? ²] + [Fe? ³]) = 1/ (1 + [Fe? ²]/ [Fe? ³]) = 1/ (1+√10) = 1/4.16 = 0.24 = 24 × 10? ²
For the reaction A + B ? 2C
The value of equilibrium constant is 100 at 298K. If the initial concentration of all the three species is 1M each, then the equilibrium concentration of C is x × 10?¹M. The value of x is ______ (Nearest integer).
A + B? 2C
Initial: 1, 1
At eq: 1-x, 1+2x
K = [C]²/ ( [A] [B]) = (1+2x)²/ (1-x)² = 100
(1+2x)/ (1-x) = 10
1+2x = 10-10x => 12x = 9 => x = 3/4
[C] = 1+2x = 1+2 (3/4) = 1+1.5 = 2.5M = 25 × 10? ¹M
A source of monochromatic radiation of wavelength 400 nm provides 1000 J of energy in 10 seconds. When this radiation falls on the surface of sodium, x × 10²? electrons are ejected per second. Assume that wavelength 400 nm is sufficient for ejection of electron from the surface of sodium metal. The value of x is ______ (Nearest integer).
(h = 6.626 × 10?³?Js)
Energy per second = 1000 J / 10 s = 100 J/s
Energy of one photon E = hc/λ = (6.626×10? ³? × 3×10? ) / (400×10? ) = 4.965 × 10? ¹? J
Number of electrons ejected = Total energy / Energy per photon = 100 / (4.965 × 10? ¹? ) = 20.14 × 10¹? ≈ 2 × 10²?
A home owner uses 4.00 × 10³m³ of methane (CH?) gas, (assume CH? is an ideal gas) in a year to heat his home. Under the pressure of 1.0 atm and 300 K, mass of gas used is x × 10?g. The value of x is ______ (Nearest integer).
(Given R = 0.083 L atm K?¹mol?¹)
PV = nRT
n = PV/RT = (1 atm × 4×10? L) / (0.083 LatmK? ¹mol? ¹ × 300 K) = 1.6 × 10? mol
Mass = n × Molar Mass = 1.6 × 10? mol × 16 g/mol = 25.6 × 10? g ≈ 26 × 10? g
Three moles of AgCl get precipitated when one mole of an octahedral co-ordination compound with empirical formula CrCl?·3NH?·3H?O reacts with excess of silver nitrate. The number of chloride ions satisfying the secondary valency of the metal ion is ______.
CrCl? ·3NH? ·3H? O gives 3 moles of AgCl precipitate. This means all three Cl? are outside the coordination sphere.
The complex is [Cr (NH? )? (H? O)? ]Cl?
The 3 chloride ions satisfy only primary valency.
secondary valency satisfied by chloride ion = 0
JEE Mains 2020
JEE Mains 2020
Chemistry NCERT Exemplar Solutions Class 11th Chapter One Exam