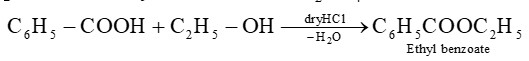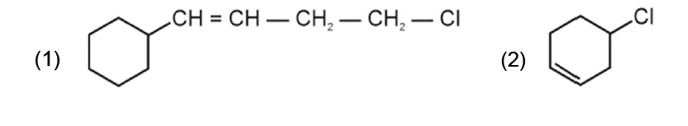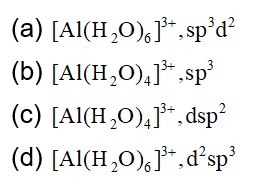Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Ethyl benzoate can be prepared by heating benzoic acid with ethyl alcohol in presence of dry HC1 or conc. The reaction is called as esterification reaction.
New answer posted
3 months agoContributor-Level 10
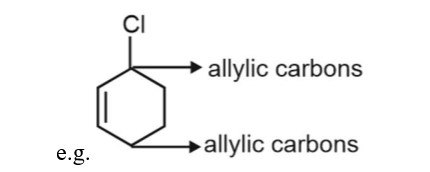
The carbon next to an alkene is known as allylic carbon and halogen attached to allylic carbon is known as allylic halogen.
New answer posted
3 months agoContributor-Level 10
Boiling points of aldehydes are higher than hydrocarbons. It is due to weak molecular association.
New answer posted
3 months agoContributor-Level 10
The colour changes due to change in pH not due to its reaction with water.
New answer posted
3 months agoContributor-Level 10
Each of the element in group III
Mn is in group seven shows a maximum oxidation state of +7
New answer posted
3 months agoContributor-Level 10
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers