Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
2 months agoContributor-Level 10
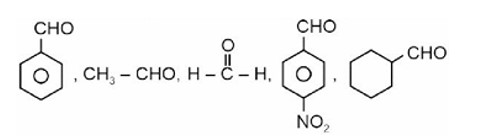
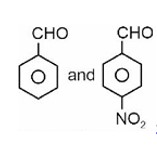
Fehling solution test can be given by aldehyde except aromatic aldehyde
New answer posted
2 months agoContributor-Level 10
Assume mass of solution
= 100g
Mass of solute = 36gm
Moles of solute = 1
Molarity=
New answer posted
2 months agoBeginner-Level 5
NCERT offers the foundation for every class 12 student. CBSE board exam questions are primarily based on the NCERT exercise, Examples and conceptual explanation.
There are various benefits of using NCERT Solutions.
- Specially for CBSE Board students, these NCERT Solutions are the best resource to prepare for the boards.
- These NCERT Solutions include step-wise descriptive answer approach that will help you write good answers.
- These solutions also help you understand problem-solving and how to approach a specific type of conceptual or numerical problem.
- If you are preparing for the JEE or NEEt exam, these solutions can be your first deci
New answer posted
2 months agoNew answer posted
2 months agoContributor-Level 10
A. Vitamin B12– Co
B. Wilkinson catalyst –Rh ( [Rh (PPh3)3Cl])
C. Ziegler-Natta catalyst –Ti (TiCl4 +Al (C2H5)3)
D. Haemoglobin – Fe
New answer posted
2 months agoContributor-Level 10
Ionisation enthalpy increases in a period. Z dominates over screening effect (s) in a period as Zeff. increases.
New answer posted
2 months agoBeginner-Level 5
Shiksha focuses on completely fulfilling the needs of CBSE class 12 board students. We provide NCERT Solutions for classes 11th and 12th in class for physics, chemistry and mathematics.
Here are the key reasons why you should use Shiksha's NCERT Solutions for the Solution Chapter:
- Complete coverage of NCERT Exercise, including MCQs, very short, short and long answer type questions.
- Step-by-step solutions according to the CBSE board exam needs.
- Intext question and answer with a free downloadable PDF
- complete study material for the chapter
- NCERT Chapter 1 Solutions Notes
- Solutions Quick Revision Notes
- Important Questions for Solution
New answer posted
2 months agoContributor-Level 10
Potassium permanganate in alkaline medium oxidise lodide to lodate.
Compound A is
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 680k Reviews
- 1800k Answers