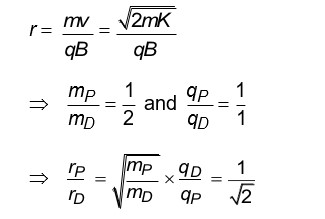Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
In reverse biasing, due to collision of electrons and atom, avalanche breakdown occurs.
New answer posted
3 months agoContributor-Level 10
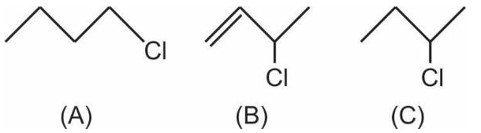
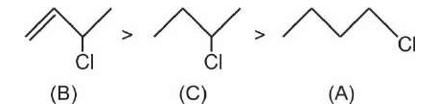
Higher is the stability of carbocation, greater is the reactivity towards SN1.
Therefore, Correct order of reactivity towards SN1 reaction is
New answer posted
3 months agoContributor-Level 10
Ranitidine, cimetidine and magnesium hydroxide are antacids.
Serotonin is a tranquilizers.
New answer posted
3 months agoContributor-Level 10
The carbonyl compounds which contain at least two a-hydrogen will give aldol condensation.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers