Electrochemistry fills the gap between chemistry and electricity. Many chemicals, such as chlorine, fluorine, sodium hydroxide, and many metals, are formed through electrochemical reactions. Not just this, but all batteries, from watch batteries to car batteries, work on electrochemical principles.
Electrochemistry class 12 covers many topics such as electrochemical cells, galvanic cells, Nernst equation, electrolysis, etc. In the class 12 electrochemistry notes, we’ve covered all the important topics from the chapter and explained each one in the simplest manner that not only helps you revise each topic quickly but also builds a better understanding of the topic.
- What is Electrochemistry?
- Electrochemical Cells
- Galvanic Cell
- Nernst Equation
- Conductance of Electrolytic Solutions
- Electrolytic Cell and Electrolysis
- Batteries
- Fuel Cells
- Corrosion
- Revision Notes for Class 12 Chemistry
- NCERT Solutions for Class 12 Chemistry
- Electrochemistry FAQs
What is Electrochemistry?
As per NCERT, “Electrochemistry is the study of the production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations”.
Simply explaining this:
Electrochemistry is the study of how chemical reactions can produce electricity or electrical energy, and this energy can drive chemical reactions that wouldn’t happen on their own.
For example, consider your phone's battery. When you use your phone, chemical reactions inside convert stored chemical energy into electricity. When you charge it, electricity is being used to reverse those chemical reactions and store energy again.
This conversion of chemical reactions into electricity and electricity to chemical energy explains what electrochemistry is all about.
Electrochemical Cells
An electrochemical cell is a device that either produces electricity from chemical reactions or uses electricity to make chemical reactions.
Every electrochemical cell has these essential parts:
- Two conducting surfaces where reactions occur called electrode
- A solution containing ions that can carry an electrical current called electrolyte
- Wires that allow electrons to flow between electrodes called external circuit.
Types of Electrochemical Cells
Electrochemical cells are of two types: a Galvanic cell and an Electrolytic cell.
Galvanic Cells
Galvanic cells convert chemical energy into electrical energy through spontaneous reactions.
Example: Your car battery, flashlight batteries
Electrolytic Cells
Electrolytic cells use external electrical energy to force non-spontaneous chemical reactions.
Example: Metal plating, water splitting for hydrogen production
Galvanic Cell
As stated earlier, Galvanic cells are the generators of electricity. They convert chemical energy into electrical energy.
The best example of a Galvanic cell is a Daniell cell, which covert chemical energy into electrical energy through a redox reaction.
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
This reaction occurs in two half-reactions:
At the anode, Zn(s) → Zn²⁺(aq) + 2e⁻ (oxidation), and
at the cathode, Cu²⁺(aq) + 2e⁻ → Cu(s) (reduction).
Electrode Potential:
Every electrode develops a potential or voltage when placed in an electrolyte solution. Because
- Metal atoms may dissolve as ions, leaving electrons on the electrode
- Metal ions from the solution may deposit on the electrode
- An equilibrium forms, creating a charge separation
Anode vs Cathode:
Anode is the part of the cell where oxidation happens or electrons are lost.
- Anode has a negative potential in galvanic cells
- Electrons flow out of the anode
The cathode is the other half of the cell where reduction happens or electrons are gained.
- The cathode has a positive potential in galvanic cells
- Electrons flow into the cathode
Standard Electrode Potentials (E°)
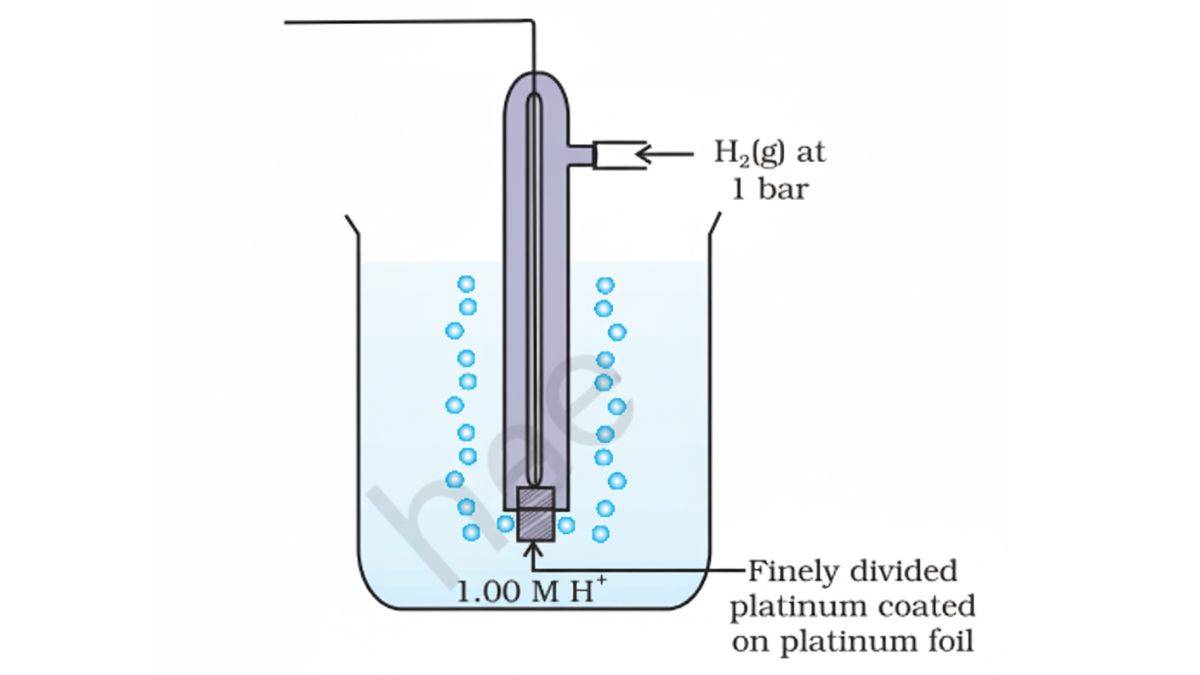
Since we can’t measure the potential or voltage of a single electrode, scientists created a reference system using the Standard Hydrogen Electrode (SHE).
- Reference potential: 0.00 V (by definition)
- Conditions: 1 M H⁺ ions, 1 bar H₂ gas, 25°C
- Reaction: 2H⁺(aq) + 2e⁻ → H₂(g)
Using the Standard Hydrogen Electrode (SHE) as reference:
- Positive E°: Species gets reduced more easily than H⁺
- Negative E°: Species gets reduced less easily than H⁺
For any galvanic cell:
E°cell = E°cathode - E°anode
Example: For the Daniell cell
- E°(Cu²⁺/Cu) = +0.34 V
- E°(Zn²⁺/Zn) = -0.76 V
- E°cell = 0.34 - (-0.76) = 1.10 V
Nernst Equation
The Nernst equation explains how cell potential (voltage) changes when conditions aren’t standard. This equation was developed by Walther Nernst, responsible for the concentration effects on cell potential.
Nernst Equation for a general reaction:
E(cell) = E°(cell) - (RT/nF) ln Q
Where:
- R: Gas constant
- T: Temperature in Kelvin
- n: Number of electrons transferred
- F: Faraday constant
- Q: Reaction quotient
Simplified Form of Nernst equation at 25°C:
At 298 K or 25°C, the equation becomes:
E(cell) = E°(cell) - (0.059/n) log Q
Equilibrium Constant from Nernst Equation
When a cell reaches equilibrium, E(cell) becomes 0 means no net current flows, and Q = K (reaction quotient becomes equal to the equilibrium constant.
This gives us: E°(cell) = (0.059/n) log K
This helps calculate equilibrium constants from standard potentials.
Connection to Gibbs Energy
The Nernst equation connects electrochemistry to thermodynamics:
ΔG = -nFE(cell)
For standard conditions:
ΔG° = -nFE°(cell)
This means:
- Positive E°: Negative ΔG°
- Negative E°: Positive ΔG°
Conductance of Electrolytic Solutions
Electricity flows through solutions through ions. In metals, electrons carry current. But in solutions, the ions carry the current.
Resistance and Conductance
- Resistance (R): Opposition to current flow (measured in ohms, Ω)
- Conductance (G): Ability to conduct current = 1/R (measured in siemens, S)
Resistivity and Conductivity
Just like how different materials have different heat resistance, they also vary in electrical resistance.
Resistance is directly proportional to the length and inversely proportional to the area of cross-section.
R = ρ(l/A)
Where,
- ρ: Resistivity
- l: Length of conductor
- A: Cross-sectional area
- Units: Ω⋅m
Conductance is the opposite of resistance
G= κ(A/l)
Where,
- κ: Conductivity
The inverse of the resistivity is called conductivity.
Conductivity (κ): κ = 1/ρ
- Units: S⋅m⁻¹ or S⋅cm⁻¹
Measurement of Conductivity of Ionic Solutions
We can measure the conductivity of ionic solutions through a conductivity cell.
Conductivity Cell
The conductivity cell contains two platinum electrodes with area A, separated by a distance l.
So the resistance will be:
R = ρ(l/A)
R = l/κA
Here,
- Cell constant: G* = l/A = Rκ
- Measurement: κ = G*/R
Molar Conductivity
Molar conductivity (Λm) is used to identify how well one mole of an electrolyte conducts electricity.
Λm = κ/c
Where.
- κ: Conductivity
- c: Molar concentration
- Units: S⋅cm²⋅mol⁻¹
Variation of Conductivity and Molar Conductivity with Concentration
Strong Electrolytes
In strong electrolytes, molar conductivity (Λm) decreases slowly with increasing concentration because of complete dissociation, but ions interfere with each other at higher concentrations
Kohlrausch equation: Λm = Λ°m - A√c
Weak Electrolytes
In weak electrolytes, molar conductivity (Λm) decreases rapidly with increasing concentration because the degree of dissociation decreases with concentration.
Need Kohlrausch law for Λ°m
Kohlrausch Law of Independent Migration
This law states that the limiting molar conductivity of an electrolyte equals the sum of individual ionic contributions.
Λ°m = n₊λ°₊ + n₋λ°₋
Where:
- λ°₊, λ°₋: Individual ionic conductivities
- n₊, n₋: Number of cations and anions
Applications of the Kohlrausch Law
- Calculate Λ°m for weak electrolytes
- Find degree of dissociation: α = Λm/Λ°m
- Calculate dissociation constants: K = α²c/(1-α)
Electrolytic Cell and Electrolysis
While galvanic cells generate electricity from chemical reactions, electrolytic cells do the opposite; they use electricity to drive chemical reactions that wouldn’t happen on their own.
In electrolytic cells, we force electrons to flow in the opposite direction.
Faraday’s Laws of Electrolysis
Michael Faraday discovered quantitative relationships governing electrolysis. Faraday’s two laws of electrolysis are:
1. The amount of chemical change is directly proportional to the quantity of electricity passed.
Chemical change ∝ Charge passed
2. Different substances liberated by the same quantity of electricity are proportional to their chemical equivalent weights.
Amount liberated ∝ (Atomic mass)/(Charge on ion)
Quantitative Relationships:
- Faraday constant (F): 96,487 C/mol (charge on 1 mole of electrons)
- For practical calculations: F ≈ 96,500 C/mol
Example: To deposit 1 mole of Cu from Cu²⁺:
- Electrons needed: 2 moles
- Charge required: 2F = 2 × 96,500 = 193,000 C
Products of Electrolysis
What gets produced during electrolysis depends on several factors:
Competing Reactions: When multiple species can be oxidized or reduced, the one with the more favorable potential wins.
At cathode (reduction):
- Most positive E° gets reduced first
Example: In aqueous NaCl, H₂O gets reduced instead of Na⁺ because H₂O reduction has a higher (less negative) potential
At anode (oxidation):
- The most negative E° gets oxidized first
Some reactions need extra voltage to occur at reasonable rates
For Example,
1. Electrolysis of molten NaCl:
- Cathode: Na⁺ + e⁻ → Na (sodium metal)
- Anode: Cl⁻ → ½Cl₂ + e⁻ (chlorine gas)
2. Electrolysis of aqueous NaCl:
- Cathode: 2H₂O + 2e⁻ → H₂ + 2OH⁻ (hydrogen gas)
- Anode: 2Cl⁻ → Cl₂ + 2e⁻ (chlorine gas)
Net result: NaOH solution, H₂ gas, and Cl₂ gas
Read more: Electrolytic Cell and Electrolysis
Batteries
Batteries are portable galvanic cells that store chemical energy and convert it to electrical energy when needed. They’re everywhere in our world, from digital watches to car batteries.
There are two kinds of batteries: Primary Batteries and Secondary Batteries.
Primary Batteries
These are like single-use items; once the chemical reactions are complete, they’re done, you can’t recharge them.
Characteristics of primary batteries:
- Single-use: Cannot be recharged
- Convenient: Ready to use immediately
- Limited life: Reactions eventually stop
- Examples: Alkaline batteries, dry cells
Secondary Batteries
These are the rechargeable batteries that can be used multiple times.
Characteristics of secondary batteries:
- Rechargeable: Can be reused with external electricity
- Reusable: Multiple charge-discharge cycles
- Higher initial cost, but economical, as it has a long life
- Examples: Car batteries, phone batteries
Fuel Cells
Corrosion
Revision Notes for Class 12 Chemistry
NCERT Solutions for Class 12 Chemistry
Electrochemistry FAQs
Commonly asked questions
What are the two branches of electrochemistry?
The two branches of electrochemistry are: Electricity generated by chemical reactions and Chemical reactions that generate electricity.
What are the two types of conductors in electrochemistry?
In electrochemistry, metallic and electrolytic are two types of conductors.
What is a Salt Bridge?
A salt bridge is a U-shaped tube that connects the two halves (oxidation and reduction) of the cell with an electrochemical cell, such as a galvanic cell. It allows the flow of ions in both halves and maintains the electricity to stay neutral.
Chemistry Electrochemistry Exam