Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
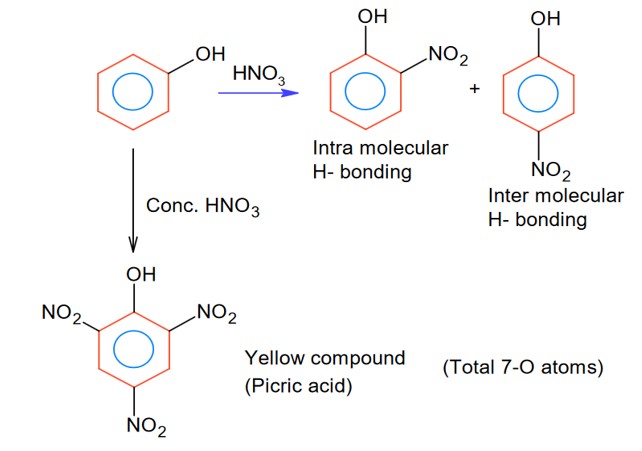
Intermolecular H- bonding and intra-molecular H- bonding producing compound may be the phenol derivatives.
New answer posted
6 months agoContributor-Level 10
E1 (done to q1) =
E2 (done to q2) =
Enet = E1 + E2
Given Enet = 6.4 * 104
d2 = 9 * 10-6 + 6
d = 3m
New answer posted
6 months agoContributor-Level 10
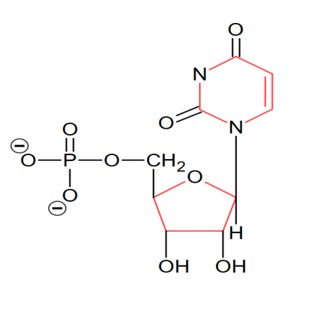
Complete combustion of compound produces 0.2 gm CO2
Hence wt of carbon in 0.2 gm CO2
Therefore % of carbon in compound
New answer posted
6 months agoContributor-Level 10
For precipitation of two moles of AgCl
Two Cl will produce as a free anion
CoCl3.4NH3 complex will Cl (will not give 2Cl)
complex will be H2 [PtCl6] will not any Cl
will produce two Cl ion.
precipitate formation
New answer posted
6 months agoContributor-Level 10
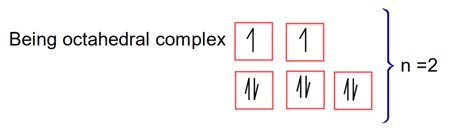
Most basic oxide V2O3
Here V has +3 O.S. Hence V+3
two unpaired e- in d- subshell
New answer posted
6 months agoContributor-Level 10
Volume of H2 adsorbed =
Therefore volume of gas adsorbed per gram of the adsorbent =
New answer posted
6 months agoContributor-Level 10
Process is based upon simultaneous disintegration hence,
………….(i)
and ………….(ii)
from equation (i) and (ii)
Here; A0 = B0 and
Therefore
New answer posted
6 months agoContributor-Level 10
2 moles of water produced by 1 mole of methane
Or 36 gm of water produced by 1 mole of CH4
81 gm of water produced = = 2.25 mole of CH4
Mole of CH4 required = 225 * 10-2
the nearest integer = 225
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers