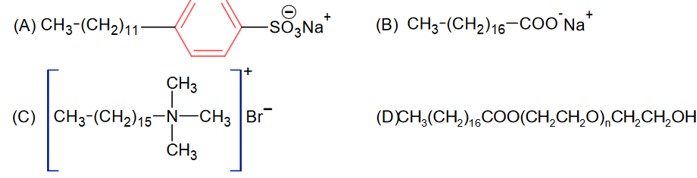Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
Buna-S is the copolymer of butadiene and styrene, Nylon-6, 6 is the condensation polymer of adipic acid and diamine and having fibrous nature.
New answer posted
6 months agoContributor-Level 10
CH3 – (CH2)16 – COONa is the sodium salt of fatty acid used in soap is not synthetic detergent.
New answer posted
6 months agoContributor-Level 10
Enamines are inter conversible and have low stability with respect to imine. Among all C is most stable due to steric factor.
New answer posted
6 months agoContributor-Level 10
p- toluenesulphonyl chloride
Only gives soluble product with alkali for 1° amine and for 2°- amine it gives insoluble product.
New answer posted
6 months agoContributor-Level 10
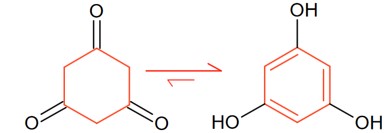
Aromaticity drives the highest enolic percentage of given structure:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers