- Solutions Questions and Answers
- 02 Oct 2025
Solutions Questions and Answers
| 1. Define the following modes of expressing the concentration of a solution. Which of these modes are independent of temperature and why? (i) (percentage mass by mass) |
| Ans: Mass percentage : It is a weight by weight relationship. Thus mathematically , Mass % of a component = where , W represents weight of the component or solution |
| (ii) (volume by volume percentage) |
| Ans: Volume percentage : Volume % of a component= where , V represents volume |
| (iii) (mass by volume percentage) |
| Ans: It is mass by volume percentage : Thus mathematically, where W is weight of solute in grams , but measures volume of solution in milliliters (ml). = |
| (iv) ppm. (parts per million) |
| Ans: Parts per million or ppm is a unit of measure of dissolved solids in solution, in terms of a ratio between the number of parts of solids/solute to a million parts of total volume. Thus, 1 Parts per million (ppm):- is a concentration of solution that contains 1 gram solute and 1000000 ml solution (same as 1 milligram solute per litre of solution) |
| (v) x, (mole fraction) |
| Ans: Mole fraction of a component (x)= |
| (vi) M (Molarity) |
| Ans: The no. of moles of solute per litre of solution. Molarity (M )= |
| (vii) m (Molality) |
| Ans: The no. of moles of solute per kg of solvent. Molality (m) = Effect of temperature: mass %, ppm, mole fraction and molality do not change with temperature whereas molarity, volume percentage and mass by volume percentage changes with temperature because volume of solution (liquid) varies with changes in temperature. |
| 2. Using Raoult’s law explain how the total vapour pressure over the solution is related to mole fraction of components in the following solutions. (i) CHCl3 (l) and CH2Cl2 (l) |
| Ans: For a binary solution having both components as volatile liquids (viz. CHCl3 and CH2Cl2), the total pressure will be p= p1= partial vapour pressure of component 1 (ie. CHCl3) p2= partial vapour pressure of component 2 (ie. CH2Cl2) |
| (ii) NaCl(s) and H2O (l) |
| Ans: For a solution containing non-volatile solute ie. NaCl (s) and H2O (l), the Raoult’s law is applicable only to vaporisable component (1) ie. H2O (l) and total vapour pressure is written as P= P1= x1P10+ x2P20 = x1P10+ (1-x1) P20 (P10-P20)X1 + P20 p= p1= partial vapour pressure of component 1 (CHCl3 ) p2= partial vapour pressure of component 2 (CH2Cl2) |
| 3. Explain the terms ideal and non-ideal solutions in the light of forces of interactions operating between molecules in liquid solutions. |
| Ans: Ideal solution: The 'ideal solution' is a binary solution of two volatile liquids that follows Raoult's rule at any concentration and temperature. Ideal solutions are formed when the intermolecular attractive forces between the solute(A) and the solvent(B) (ie.A-B interaction) are approximately equal to those between the solvent-solvent (A-A) and the solute-solute (BB). Enthalpy of mixing, mixing H=0, in such a perfect solution. Volume change on mixing, Δ mixing V=0. Examples: n- hexane and n-heptane. Non ideal solution: At any concentration and temperature, these binary solutions of two volatile liquids do not obey Raoult's law. PA≠PoAxA PB≠PoBxB Furthermore, non-ideal solutions are formed when the intermolecular attractive forces between the solute and the solvent (A-B interaction) are not equal (either stronger or weaker) to those between the solvent and the solvent (A-A) and the solute and the solute (B-B). Enthalpy of mixing, ΔH is not equal to 0. & Volume change on mixing, V is not equal to 0. Example: CS2 and acetone. where,PoA, PoB denote the vapour pressures of pure solvent and PA, PB denote the partial vapour pressures of components A and B in solution, and X denotes the mole fractions of the two components denoted by the subscripts A and B. |
| 4. Why is it not possible to obtain pure ethanol by fractional distillation? What general name is given to binary mixtures which show deviation from Raoult’s law and whose components cannot be separated by fractional distillation. How many types of such mixtures are there? |
| Ans: "Azeotropes” is the general term for binary mixes that deviate from Rault's law and whose components cannot be separated by fractional distillation. Because of the following reasons, fractional distillation cannot produce pure ethanol: Azeotropes are binary solutions (liquid mixes) with the same composition in the liquid and vapour phases, therefore fractional distillation cannot separate the components of an azeotrope. On fractional distillation, an ethanol-water mixture (obtained via sugar fermentation) yields a solution containing approximately 95 percent ethanol by volume of ethanol. Because the liquid and vapour phases have the identical composition, they cannot be separated. There are two sorts of binary mixtures that are referred to as- (1) Minimum boiling azeotrope: At a given composition, non-ideal solutions show a considerable positive divergence from the minimal boiling azeotrope. For instance, 95 percent ethanol and 5% water (by volume): Below are the boiling points of pure ethanol, water, and its azeotrope. Ethanol has a melting point of 351.3 degrees Celsius, while water has a melting point of 373 degrees Celsius and azeotrope has a melting point of 351.1 degrees Celsius. (2) Maximum boiling azeotrope: Non-ideal solutions with a considerable negative departure from Rault's law from the maximum boiling azeotrope at a given composition. By mass, nitric acid and water have a composition of 68 percent nitric acid and 32 percent water. The boiling point of such an azeotropic HNO3-H2O mixture is 393.5 K. |
Commonly asked questions
On the basis of information given below mark the correct option. Information:
(A) In bromoethane and chloroethane mixture intermolecular interactions of A–A and B–B type are nearly same as A–B type interactions.
(B) In ethanol and acetone mixture A–A or B–B type intermolecular interactions are stronger than A–B type interactions.
(C) In chloroform and acetone mixture A–A or B–B type intermolecular interactions are weaker than A–B type interactions.
(i) Solution (B) and (C) will follow Raoult’s law.
(ii) Solution (A) will follow Raoult’s law.
(iii) Solution (B) will show negative deviation from Raoult’s law.
(iv) Solution (C) will show positive deviation from Raoult’s law.
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Solution (A) will follow Raoult's law.
Explanation: A-A and B-B intermolecular interactions should be almost identical to A-B type interactions in an ideal solution.
When kept in water, raisin swells in size. Name and explain the phenomenon involved with the help of a diagram. Give three applications of the phenomenon.
This is a long answer type question as classified in NCERT Exemplar
Raised increases in size when submerged in water. It's due to a phenomenon known as "Osmosis." The process is depicted graphically in figure. A semipermeable membrane separates a solution from its solvent in this procedure, allowing solvent molecules to pass through but preventing solute particles from passing through.
The passage of solvent molecules from a pure solvent to a solution via a semipermeable membrane is known as osmosis. The following are three osmosis applications:
(i) Osmosis is responsible for some of the water movement from the soil into the plant roots and then into the top parts of the plant.
(ii) Adding salt to meat to protect it from bacterial attack.
(iii) Adding sugar to fruits to protect them from bacterial attack. Bacterium in canned fruit shrivels and dies as a result of the osmosis process.
(a) Explain the following phenomena with the help of Henry’s law.
(i) Painful condition known as bends.
(ii) At high altitude, a feeling of weakness and difficulty breathing.
(b) Why soda water bottle kept at room temperature fizzes on opening?
This is a short answer type question as classified in NCERT Exemplar
(a) (i) According to Henry's law, a gas's pressure is proportional to its solubility. The air pressure gradually falls as scuba divers approach the surface. This lower pressure causes the dissolved gases in the blood to be released, resulting in the development of nitrogen bubbles in the blood. This causes capillaries to constrict, resulting in bends, a painful and life-threatening medical condition.
(ii) The partial pressure of oxygen at high altitude is lower than at ground level. People living at high altitudes have reduced oxygen concentrations in their blood and tissues as a result of this. Low blood oxygen causes weakness and discomfort.
(b)When a room-temperature soda water bottle is opened to the air, the partial pressure of CO2 above the solution drops dramatically (as per Henry's law). As a result, the solubility of carbon dioxide decreases, causing CO2 bubbles to fizz out of the bottle.
Low concentration of oxygen in the blood and tissues of people living at high altitude is due to ____________.
(i) Low temperature
(ii) Low atmospheric pressure
(iii) High atmospheric pressure
(iv) Both low temperature and high atmospheric pressure
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Low atmospheric pressure
Explanation: The atmospheric pressure drops at high altitude, reducing the solubility of oxygen in blood and tissues.
On dissolving sugar in water at room temperature solution feels cool to touch. Under which of the following cases dissolution of sugar will be most rapid?
(i) Sugar crystals in cold water.
(ii) Sugar crystals in hot water.
(iii) Powdered sugar in cold water.
(iv) Powdered sugar in hot water.
This is a multiple choice answer as classified in NCERT Exemplar
(iv) Powdered sugar in hot water.
Explanation: (i) The solubility of a solute/sugar particle increases as its surface area increases. Powdered sugar dissolves more quickly due to its increased surface area.
(ii) Because it provides a cool sensation when touched, sugar dissolving is an endothermic process. Increasing the temperature or using hot water, according to LeChatelier's theory, facilitates sugar breakdown.
At equilibrium the rate of dissolution of a solid solute in a volatile liquid solvent is ________.
(i) Less than the rate of crystallisation.
(ii) Greater than the rate of crystallisation
(iii) Equal to the rate of crystallisation
(iv) Zero
This is a multiple choice answer as classified in NCERT Exemplar
(iii) Equal to the rate of crystallisation
Explanation : This occurs under equilibrium conditions, i.e. rate of forward reaction (dissolution) = rate of backward reaction (crystallisation).
A beaker contains a solution of substance ‘A’. Precipitation of substance ‘A’ takes place when small amount of ‘A’ is added to the solution. The solution is_________.
(i) Saturated
(ii) Supersaturated
(iii) Unsaturated
(iv) Concentrated
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Supersaturated
Explanation: At a certain temperature, a supersaturated solution contains less than the maximum quantity of solute per given amount of solvent. When a small amount of solute is added to a solution like this, it precipitates/crystallizes quickly. A supersaturated solution varies from an unsaturated solution in that it does not precipitate or crystallise when a tiny amount of solute is added to it; instead, it goes into solution and remains dissolved at a specific temperature.
Maximum amount of a solid solute that can be dissolved in a specified amount of a given liquid solvent does not depend upon ____________.
(i) Temperature
(ii) Nature of solute
(iii) Pressure
(iv) Nature of solvent
This is a multiple choice answer as classified in NCERT Exemplar
(iii) Pressure
Explanation: Because solids and liquids are nearly incompressible, the solubility of a solid in a liquid is unaffected by pressure.
Considering the formation, breaking and strength of hydrogen bond, predict which of the following mixtures will show a positive deviation from Raoult’s law?
(i) Methanol and acetone.
(ii) Chloroform and acetone.
(iii) Nitric acid and water.
(iv) Phenol and aniline.
This is a multiple choice answer as classified in NCERT Exemplar
(i) Methanol and acetone.
Explanation: The interaction (A-A)* is greater than the interaction (A-B)*. Methanol has greater intermolecular hydrogen bonding than methanol and acetone combined. As a result, methanol and acetone mixes will deviate from Raoult's law in a favourable way. The (A-A)* interaction is the interaction of acetone particles/molecules that do not have any hydrogen bonds between them.
The interaction between the particles / molecules of acetone and methanol is known as the (A-B)* interaction.
Colligative properties depend on ____________.
(i) The nature of the solute particles dissolved in solution.
(ii) The number of solute particles in solution.
(iii) The physical properties of the solute particles dissolved in solution.
(iv) The nature of solvent particles.
This is a multiple choice answer as classified in NCERT Exemplar
(ii) The number of solute particles in solution.
Which of the following aqueous solutions should have the highest boiling point?
(i) 1.0 M NaOH
(ii) 1.0 M Na2SO4
(iii) 1.0 M NH4NO3
(iv) 1.0 M KNO3
(ii) 1.0 M Na2SO4
Explanation: When compared to the other three electrolytes, the van't Hoff factor in 1.0 M Na2SO4 solution is I > 1 and is the highest. As a result, when compared to the other electrolytes in their 1.0 M solutions, the extent of dissociation in the case of 1.0 M Na2SO4 would be the greatest, giving the greatest number of ions.
Value of Henry’s constant KH ____________.
(i) Increases with increase in temperature.
(ii) Decreases with increase in temperature.
(iii) Remains constant.
(iv) First increases then decreases.
This is a multiple choice answer as classified in NCERT Exemplar
(i) Increases with increase in temperature.
The value of Henry’s constant KH is_____________.
(i) Greater for gases with higher solubility.
(ii) Greater for gases with lower solubility.
(iii) Constant for all gases.
(iv) Not related to the solubility of gases.
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Greater for gases with lower solubility.
Explanation : Because of the mathematical relationship, the value of Henry's constant KH is bigger for gases with lesser solubility-
p= kH x
kH=p/x
Where, KH represents Henry's constant, p is partial pressure of the gas in vapour phase, and x denotes mole fraction of the gas in solution. Thus KH is inversely proportional to mole fraction of gas in solution (representing its solubility)
Consider the Fig. 2.1 and mark the correct option.
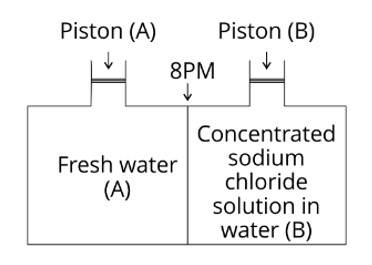
(i) Water will move from side (A) to side(B) if a pressure lower than osmotic pressure is applied on piston (B).
(ii) Water will move from side (B) to side (A) if a pressure greater than osmotic pressure is applied on piston (B).
(iii) Water will move from side (B) to side (A) if a pressure equal to osmotic pressure is applied on piston (B).
(iv) Water will move from side (A) to side (B) if pressure equal to osmotic pressure is applied on piston (A).
This is a multiple choice answer as classified in NCERT Exemplar
(ii) Water will move from side (B) to side (A) if a pressure greater than osmotic pressure is applied on piston (B).
Explanation: Due to reverse osmosis, water will travel from side (B) to side (A) if a pressure greater than osmotic pressure is applied to the piston (B).
Two beakers of capacity 500 mL were taken. One of these beakers, labelled as “A”, was filled with 400 mL water whereas the beaker labelled “B” was filled with 400 mL of 2 M solution of NaCl. At the same temperature both the beakers were placed in closed containers of same material and same capacity as shown in Fig.2.2. At a given temperature, which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution.

(i) Vapour pressure in container (A) is more than that in container (B).
(ii) Vapour pressure in container (A) is less than that in container (B).
(iii) Vapour pressure is equal in both the containers.
(iv) Vapour pressure in container (B) is twice the vapour pressure in container (A).
This is a multiple choice answer as classified in NCERT Exemplar
(i) Vapour pressure in container (A) is more than that in container (B).
Explanation: Due to the fleeing inclinations of water molecules from the liquid's surface, the vapour pressure rises. The vapour pressure increases as the number of molecules on the liquid's surface increases. Because only water molecules are present at the surface of beaker A, it has a higher vapour pressure. However, a fraction of the surface area of the solution in beaker B containing NaCl solution is occupied by NaCl molecules, which are non-volatile and have no tendency to escape. As a result, the quantity of water molecules present in NaCl solution decreases, lowering its vapour pressure.
If two liquids A and B form minimum boiling azeotrope at some specific composition then_______________.
(i) A–B interactions are stronger than those between A–A or B–B.
(ii) Vapour pressure of solution increases because more number of molecules of liquids A and B can escape from the solution.
(iii) Vapour pressure of solution decreases because less number of molecules of only one of the liquids escapes from the solution.
(iv) A–B interactions are weaker than those between A–A or B–B.
This is a multiple choice answer as classified in NCERT Exemplar
(iv) A–B interactions are weaker than those between A–A or B–B.
Explanation: (i) At a given composition, the solutions that demonstrate a big positive divergence from Rault's law form a minimum boiling azeotrope.
(ii) When Rault's law is deviated positively, A-B interactions are weaker than A-A or B-B interactions.
4L of 0.02 M aqueous solution of NaCl was diluted by adding one litre of water. The molality of the resultant solution is _____________.
(i) 0.004
(ii) 0.008
(iii) 0.012
(iv) 0.016
This is a multiple choice answer as classified in NCERT Exemplar
(iv) 0.016
Explanation: Apply the relation : M1V1= M2V2
Given: M1=0.02M, V1=4L, M2=? V2=5L
Therefore, 0.02×4L=M2×5L
M2=0.08/5
=0.016 M
For a binary ideal liquid solution, the variation in total vapour pressure versus composition of solution is given by which of the curves?
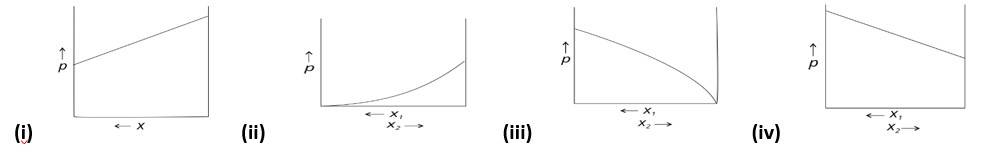
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (iv)
Explanation : Because the slopes in (i) and (iv) are straight lines, they represent the solution's ideal behaviour.
Define the following modes of expressing the concentration of a solution. Which of these modes are independent of temperature and why?
(i) (percentage mass by mass)
(ii) (volume by volume percentage)
(iii) (mass by volume percentage)
(iv) ppm. (parts per million)
(v) x, (mole fraction)
(vi) M (Molarity)
(vii) m (Molality)
This is a long answer type question as classified in NCERT Exemplar
(i)Mass percentage : It is a weight by weight relationship. Thus mathematically ,
Mass % of a component =
where , W represents weight of the component or solution
(ii) Volume percentage :
Volume % of a component=
where , V represents volume
(iii) It is mass by volume percentage : Thus mathematically, where W is weight of solute in grams , but measures volume of solution in milliliters (ml).
=
(iv) Parts per million or ppm is a unit of measure of dissolved solids in solution, in terms of a ratio between the number of parts of solids/solute to a million parts of total volume. Thus, 1 Parts per million (ppm):- is a concentration of solution that contains 1 gram solute and 1000000 ml solution (same as 1 milligram solute per litre of solution)
(v) Mole fraction of a component (x)=
(vi) The no. of moles of solute per litre of solution.
Molarity (M )=
(vii) The no. of moles of solute per kg of solvent.
Molality (m) =
Effect of temperature: mass %, ppm, mole fraction and molality do not change with temperature whereas molarity, volume percentage and mass by volume percentage changes with temperature because volume of solution (liquid) varies with changes in temperature.
Using Raoult’s law explain how the total vapour pressure over the solution is related to mole fraction of components in the following solutions.
(i) CHCl3 (l) and CH2Cl2 (l)
(ii) NaCl(s) and H2O (l)
This is a long answer type question as classified in NCERT Exemplar
(i) For a binary solution having both components as volatile liquids (viz. CHCl3 and CH2Cl2), the total pressure will be
p=
p1= partial vapour pressure of component 1 (ie. CHCl3)
p2= partial vapour pressure of component 2 (ie. CH2Cl2)
(ii) For a solution containing non-volatile solute ie. NaCl (s) and H2O (l), the Raoult’s law is applicable only to vaporisable component (1) ie. H2O (l) and total vapour pressure is written as
P= P1= x1P10+ x2P20
= x1P10+ (1-x1) P20
(P10-P20)X1 + P20
p=
p1= partial vapour pressure of component 1 (CHCl3 )
Explain the terms ideal and non-ideal solutions in the light of forces of interactions operating between molecules in liquid solutions.
This is a long answer type question as classified in NCERT Exemplar
Ideal solution: The 'ideal solution' is a binary solution of two volatile liquids that follows Raoult's rule at any concentration and temperature.
Ideal solutions are formed when the intermolecular attractive forces between the solute (A) and the solvent (B) (ie.A-B interaction) are approximately equal to those between the solvent-solvent (A-A) and the solute-solute (BB). Enthalpy of mixing, mixing H=0, in such a perfect solution.
Volume change on mixing, Δ mixing V=0.
Examples: n- hexane and n-heptane.
Non ideal solution: At any concentration and temperature, these binary solutions of two volatile liquids do not obey Raoult's law.
PA≠PoAxA
PB≠PoBxB
Furthermore, non-ideal solutions are formed when the intermolecular attractive forces between the solute and the solvent (A-B interaction) are not equal (either stronger or weaker) to those between the solvent and the solvent (A-A) and the solute and the solute (B-B).
Enthalpy of mixing, ΔH is not equal to 0. & Volume change on mixing, V is not equal to 0.
Example: CS2 and acetone.
where, PoA, PoB denote the vapour pressures of pure solvent and PA, PB denote the partial vapour pressures of components A and B in solution, and X denotes the mole fractions of the two components denoted by the subscripts A and B.
Why is it not possible to obtain pure ethanol by fractional distillation? What general name is given to binary mixtures which show deviation from Raoult’s law and whose components cannot be separated by fractional distillation. How many types of such mixtures are there?
This is a long answer type question as classified in NCERT Exemplar
"Azeotropes” is the general term for binary mixes that deviate from Rault's law and whose components cannot be separated by fractional distillation. Because of the following reasons, fractional distillation cannot produce pure ethanol: Azeotropes are binary solutions (liquid mixes) with the same composition in the liquid and vapour phases, therefore fractional distillation cannot separate the components of an azeotrope. On fractional distillation, an ethanol-water mixture (obtained via sugar fermentation) yields a solution containing approximately 95 percent ethanol by volume of ethanol. Because the liquid and vapour phases have the identical composition, they cannot be separated. There are two sorts of binary mixtures that are referred to as-
(1) Minimum boiling azeotrope: At a given composition, non-ideal solutions show a considerable positive divergence from the minimal boiling azeotrope. For instance, 95 percent ethanol and 5% water (by volume): Below are the boiling points of pure ethanol, water, and its azeotrope. Ethanol has a melting point of 351.3 degrees Celsius, while water has a melting point of 373 degrees Celsius and azeotrope has a melting point of 351.1 degrees Celsius.
(2) Maximum boiling azeotrope: Non-ideal solutions with a considerable negative departure from Rault's law from the maximum boiling azeotrope at a given composition. By mass, nitric acid and water have a composition of 68 percent nitric acid and 32 percent water. The boiling point of such an azeotropic HNO3-H2O mixture is 393.5 K.
Discuss biological and industrial importance of osmosis.
This is a long answer type question as classified in NCERT Exemplar
The process of osmosis is of immense biological and industrial importance as is evident from the following examples:
A. Biological Importance :
(i) Osmosis is responsible for some of the water movement from the soil into the plant roots and then into the top parts of the plant.
(ii) Adding salt to meat to protect it against bacterial action (ie. salting).
(iii) Sugar is used to protect fruits from bacterial attack. Bacterium in canned fruit shrivels and dies as a result of the osmosis process.
(iv) Salt blood cells break due to osmosis when placed in water containing less than 0.9 percent (mass by volume). Osmosis causes water retention in tissue cells and intercellular gaps in those who consume a lot of salt or eat salty foods. Edema is the name given to the puffiness or swelling that results as a result of this.
(v) When withered flowers are immersed in freshwater, they revive.
B. Industrial importance
(i) For desalination of seawater, reverse osmosis is used: when a pressure greater than osmotic pressure is applied to saltwater, clean water is squeezed out through a semipermeable membrane. For this, a number of semipermeable membranes are available.
How can you remove the hard calcium carbonate layer of the egg without damaging its semipermeable membrane? Can this egg be inserted into a bottle with a narrow neck without distorting its shape? Explain the process involved.
This is a long answer type question as classified in NCERT Exemplar
(i) When egg is placed in a dilute mineral acid solution (preferably dilute HCl solution), the hard external CaCO3 layer of the egg dissolves out /removed without damaging its semipermeable membrane.
(ii) Yes, this egg can be inserted into a bottle with a narrow neck without distorting in shape. The process involved utilising phenomenon of osmosis is explained as below -
Egg is placed in a mineral acid solution – after some time the egg is removed and placed in a hypertonic solution- size of the egg gradually decreases after some time and it shrivels due to osmosis. Since the egg has shrivelled it can now be inserted easily into a bottle with a narrow mouth. The egg is, therefore, placed in a bottle with a narrow neck & then a hypotonic solution is filled into this bottle. On adding hypotonic solution, the egg regains shape due to osmosis.
Hypertonic solution- is a solution with higher salt concentration than that of the normal body cells so that the solvent/water is drawn out of the cell by osmosis or any solution with higher osmotic pressure than another solution is called "Hypertonic solution".
Hypotonic solution is a solution with lower salt concentration than that of the normal body cells so that water/solvent flows into the cell by osmosis or hypotonic solution is a solution which has lower osmotic pressure than the other solution.
Why is the mass determined by measuring a colligative property in case of some solutes abnormal? Discuss it with the help of Van’t Hoff factor.
This is a long answer type question as classified in NCERT Exemplar
When dissolved in suitable solvents, certain solutes/compounds either dissociate or associate. For instance, ethanoic acid dimerises in benzene due to hydrogen bonding, but dissociates and produces ions in water.
As a result, the number of chemical species in solution rises or falls in relation to the number of chemical species of solute used to create the solution. Because the magnitude of the colligative property is dependent on the number of solute particles in relation to the total number of particles in solution, it is expected that the molar mass calculated using colligative properties will be either higher or lower than the expected or normal value, and this is referred to as abnormal molar mass.
Van't Hoff established a factor called the Van't Hoff factor to account for the degree of dissociation or association of molecules in solution. It can be summed up as follows:
The predicted colligative qualities are achieved by assuming that the nonvolatile solute is neither associated nor dissociated, and anomalous molar mass is the empirically determined molar mass. The value of I (van't Hoff factor) is smaller than unity in the event of association, but greater than unity in the case of dissociation.
Components of a binary mixture of two liquids A and B were being separated by distillation. After some time separation of components stopped and composition of vapour phase became the same as that of the liquid phase. Both the components started coming in the distillate. Explain why this happened.
Because both components exist in the distillate and the liquid and vapour compositions are the same, this indicates that the liquids have formed an azeotropic combination that cannot be separated at this stage by fractional distillation.
Explain why on addition of 1 mol of NaCl to 1 litre of water, the boiling point of water increases, while addition of 1 mol of methyl alcohol to one litre of water decreases its boiling point.
This is a short answer type question as classified in NCERT Exemplar
The vapour pressure of a liquid in comparison to air pressure determines its boiling point. At a constant atmospheric pressure, the lower the vapour pressure, the higher the boiling point of a liquid, and vice versa.
Because NaCl is a nonvolatile solute, it reduces the vapour pressure of water when added to it. The boiling point of water rises as a result. Methyl alcohol, on the other hand, is more volatile than water, therefore adding it to the solution raises the overall vapour pressure, lowering the boiling point of water.
Explain the solubility rule “like dissolves like'' in terms of inter-molecular forces that exist in solutions.
This is a short answer type question as classified in NCERT Exemplar
The solubility rule "like dissolves like" is based on the intermolecular forces of that exist in solution as follows:
If the intermolecular interactions in both components are similar, a substance (solute) dissolves in a solvent (ie. solvent and solute particles or molecules). When polar solutes dissolve in polar solvents and non-polar solutes dissolve in non-polar solvents, this is a regular occurrence.
Concentration terms such as mass percentage, ppm, mole fraction and molality are independent of temperature, however molarity is a function of temperature. Explain.
This is a short answer type question as classified in NCERT Exemplar
The number of moles of solute dissolved in one litre of solution is the molarity of a solution, which is defined as "the number of moles of solute dissolved in one litre of solution." Because volume is affected by temperature and changes with it, the molarity will also change as the temperature changes.
Other concentration words, such as mass percentage, ppm, mole fraction, and molality, are based on the mass-to-mass relationship of the solute and solvent in a binary solution. Because mass does not vary as a function of temperature, these concentration terms do not change as a function of temperature. The mass of the solvent used to make the solution is connected to the mass of the solute, according to the definitions of all of these words.
What is the significance of Henry’s Law constant KH?
This is a short answer type question as classified in NCERT Exemplar
p =KHx (where p is the partial pressure of the gas in the vapour phase and x is the mole fraction of the gas in solution) is Henry's law expressed mathematically.
As a result of the aforementioned equation, "the lower the solubility of the gas in the liquid, the greater the value of Henry's law constant KH at a given pressure."
Why are aquatic species more comfortable in cold water in comparison to warm water?
This is a short answer type question as classified in NCERT Exemplar
It's worth noting that when the temperature drops, the values of Henry's law constant (KH) rise. Because of this, the solubility of oxygen in water increases with decreasing temperature at a given pressure. As a result, the presence of more oxygen at lower temperatures makes aquatic organisms feel more at ease in cold water than in warm water.
Why is the vapour pressure of an aqueous solution of glucose lower than that of water?
This is a short answer type question as classified in NCERT Exemplar
The fleeing tendencies of water molecules from the liquid level/surface create vapour pressure in any solvent or water. Only water molecules are present at the surface of pure water, but when a nonvolatile solute such as glucose is dissolved in it, a certain number of nonvolatile glucose molecules with no escape tendency are also present at the aqueous solution's surface.
As a result, the quantity of water molecules near the surface decreases, resulting in a lower number of water molecules being able to escape as vapours. When compared to pure water/solvent, this lowers the vapour pressure of water in its glucose solution. Its colligative feature refers to this relative decrease in vapour pressure.
How does sprinkling of salt help in clearing the snow-covered roads in hilly areas? Explain the phenomenon involved in the process.
This is a short answer type question as classified in NCERT Exemplar
'Depression in the freezing point of water when a nonvolatile solute is dissolved in it' is the phenomenon involved in removing snow-covered roads in hilly places. As a result, when salt is spread over snow-covered roads, snow melts from the surface due to a drop in the freezing point of water, which aids in road cleaning.
What is “semi permeable membrane”?
This is a short answer type question as classified in NCERT Exemplar
Semi permeable membranes are continuous sheets or films (natural or synthetic) that have a network of submicroscopic holes or pores through which small solvent molecules like water may pass but larger molecules of solute cannot. Osmosis is the process of diffusion via this membrane.
Give an example of a material used for making semipermeable membrane for carrying out reverse osmosis.
This is a short answer type question as classified in NCERT Exemplar
"A sheet of cellulose acetate laid over a suitable support" is the material used to make a semipermeable membrane for reverse osmosis.
Which of the following units is useful in relating concentration of solution with its Vapour pressure?
(i) Mole fraction
(ii) Parts per million
(iii) Mass percentage
(iv) Molality
This is a multiple choice answer as classified in NCERT Exemplar
(i) Mole fraction
Explanation: The mole fraction (x) is important for determining the relationship between a solution's concentration and its vapour pressure. Rault's law states that p1 = x1 po1 in a binary solution of two volatile liquids, where p1 is the vapour pressure of component 1, x1 is its mole fraction in solution, and po1 is the vapour pressure of pure solvent.
The unit of ebullioscopic constant is _______________.
(i) Kkg mol–1 or K(molality)-1
(ii) mol kg K–1 or K–1(molality)
(iii) kg mol–1 K–1 or K–1(molality)–1
(iv) K mol kg–1 or K(molality)
This is a multiple choice answer as classified in NCERT Exemplar
(i) Kkg mol–1 or K (molality)-1
In comparison to a 0.01 M solution of glucose, the depression in freezing point of a 0.01 M MgCl2 solution is _____________.
(i) The same
(ii) About twice
(iii) About three times
(iv) About six times
This is a multiple choice answer as classified in NCERT Exemplar
(iii) About three times
Explanation: A colligative property is a decrease in freezing point. In the case of MgCl2, the van't Hoff factor will be higher. When a molecule of MgCl2 dissociates in its aqueous solution, it produces 3 ions. One molecule of 0.01M MgCl2 produces three particles/ions in solution, resulting in a threefold increase in the number of particles present in the solution. As a result, the freezing point of 0.01M MgCl2 will be three times lower than that of 0.01M glucose solution, because there will be no dissociation of the molecule.
An unripe mango placed in a concentrated salt solution to prepare pickle, shrivels because _____________.
(i) It gains water due to osmosis.
(ii) It loses water due to reverse osmosis.
(iii) It gains water due to reverse osmosis.
(iv) It loses water due to osmosis.
This is a multiple choice answer as classified in NCERT Exemplar
(iv) It loses water due to osmosis.
At a given temperature, osmotic pressure of a concentrated solution of a substance _____________.
(i) Is higher than that at a dilute solution.
(ii) Is lower than that of a dilute solution.
(iii) Is same as that of a dilute solution.
(iv) Cannot be compared with osmotic pressure of dilute solution.
This is a multiple choice answer as classified in NCERT Exemplar
(i) Is higher than that at a dilute solution.
Which of the following statements is false?
(i) Two different solutions of sucrose of same molality prepared in different solvents will have the same depression in freezing point.
(ii) The osmotic pressure of a solution is given by the equation (where C is the
molarity of the solution).
(iii) Decreasing order of osmotic pressure for 0.01 M aqueous solutions of barium chloride, potassium chloride, acetic acid and sucrose is BaCl2>KCl>CH3COOH > sucrose.
(iv) According to Raoult’s law, the vapour pressure exerted by a volatile component of a solution is directly proportional to its mole fraction in the solution.
This is a multiple choice answer as classified in NCERT Exemplar
(i) Two different solutions of sucrose of the same molality prepared in different solvents will have the same depression in freezing point.
Explanation: Tf = Kf m
The dip in freezing the point of the solution would not be the same since Kf values are dependent on the composition of the solvent.
The values of van’t Hoff factors for KCl, NaCl and K2SO4 respectively, are ______.
(i) 2, 2 and 2
(ii) 2, 2 and 3
(iii) 1, 1 and 2
(iv) 1, 1 and 1
This is a multiple choice answer as classified in NCERT Exemplar
(ii) 2, 2 and 3
Explanation: The van't Hoff factor's values are determined by the degree of dissociation. Strong electrolytes include KCl, NaCl, and K2SO4. When compared to KCl and NaCl, the extent or degree of dissociation with Na2SO4 is the largest.
Which of the following statements is false?
(i) Units of atmospheric pressure and osmotic pressure are the same.
(ii) In reverse osmosis, solvent molecules move through a semipermeable membrane from a region of lower concentration of solute to a region of higher concentration.
(iii) The value of molal depression constant depends on nature of solvent.
(iv) Relative lowering of vapour pressure, is a dimensionless quantity.
This is a multiple choice answer as classified in NCERT Exemplar
(ii) In reverse osmosis, solvent molecules move through a semipermeable membrane from a region of lower concentration of solute to a region of higher concentration.
Explanation:
Solvent molecules pass through a semipermeable membrane in reverse osmosis from an area of higher solute concentration to a region of lower concentration, hence the supplied assertion at (ii) is untrue.
On the basis of information given below mark the correct option.
Information: On adding acetone to methanol some of the hydrogen bonds between methanol molecules break.
(i) At specific composition methanol-acetone mixture will form minimum boiling azeotrope and will show positive deviation from Raoult’s law.
(ii) At specific composition methanol-acetone mixture forms maximum boiling azeotrope and will show positive deviation from Raoult’s law.
(iii) At specific composition methanol-acetone mixture will form minimum boiling azeotrope and will show negative deviation from Raoult’s law.
(iv) At specific composition methanol-acetone mixture will form maximum boiling azeotrope and will show negative deviation from Raoult’s law.
This is a multiple choice answer as classified in NCERT Exemplar
(i) At specific composition methanol-acetone mixture will form minimum boiling azeotrope and will show positive deviation from Raoult's law.
Explanation: (i) (A-A) or (B-B) interactions are more powerful than (A-B) interactions, where A represents a methanol molecule and B represents an acetone molecule. It means that molecules of A (or B) will have an easier time escaping from this solution. This will result in a positive departure from Rault's law when the vapour pressure rises.
(ii) The methanol-acetone mixture forms the smallest boiling azeotrope due to this positive divergence.
KH value for Ar(g), CO2 (g), HCHO (g) and CH4 (g) are 40.39, 1.67, 1.83×10-5 and 0.413 respectively. Arrange these gases in the order of their increasing solubility.
(i) HCHO4
(ii) HCHO2< CH4
(iii) Ar2
(iv) Ar4
This is a multiple choice answer as classified in NCERT Exemplar
(iii) Ar< CO2< CH4< HCHO
Explanation: The higher the KH value, the better. The solubility of a gas at a given pressure will be lower, hence the solubility of given gases will increase as KH values rise.
Intermolecular forces between two benzene molecules are nearly of same strength as those between two toluene molecules. For a mixture of benzene and toluene, which of the following are not true?
(i) Δmix H= zero
(ii) Δmix V= zero
(iii) These will form minimum boiling azeotrope.
(iv) These will not form ideal solution.
This is a multiple choice answer as classified in NCERT Exemplar
(iii) and (iv)
Explanation: Intermolecular forces between benzene and toluene molecules in a combination of benzene and toluene molecules would be approximately as strong as those between two benzene molecules and two toluene molecules separately. As a result, the solution will form an ideal solution and follow Raoult's law. As a result, options (iii) and (iv) are false.
Relative lowering of vapour pressure is a colligative property because _________.
(i) It depends on the concentration of a non-electrolyte solute in solution and does not depend on the nature of the solute molecules.
(ii) It depends on number of particles of electrolyte solute in solution and does not depend on the nature of the solute particles.
(iii) It depends on the concentration of a non-electrolyte solute in solution as well as on the nature of the solute molecules.
(iv) It depends on the concentration of an electrolyte or non-electrolyte solute in solution as well as on the nature of solute molecules.
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (ii)
Explanation: Colligative property depends on
(i) The concentration of a nonelectrolyte solute in solution,
(ii) The number of particles of electrolyte solute in solution, & (iii) It does not depend on the nature of solute molecules/particles.
Isotonic solutions must have the same_____________.
(i) Solute
(ii) Density
(iii) Elevation in boiling point
(iv) Depression in freezing point
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (ii)
Isotonic solutions must have same osmotic pressure at a given temperature hence must have same volume and number of moles i.e., same molar concentration. Thus, the isotonic solutions have same elevation in boiling point, and depression in freezing point.
Which of the following binary mixtures will have same composition in liquid and vapour phase?
(i) Benzene-Toluene
(ii) Water-Nitric acid
(iii) Water-Ethanol
(iv) n-Hexane-n-Heptane
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (iii)
Explanation: At a specific composition Azeotropic mixtures of water, nitric acid, and ethanol have the same composition in the vapour and liquid phases.
In isotonic solutions ________________.
(i) Solute and solvent both are the same.
(ii) Osmotic pressure is the same.
(iii) Solute and solvent may or may not be same.
(iv) Solute is always same solvent may be different.
This is a multiple choice answer as classified in NCERT Exemplar
(ii) and (iii)
For isotonic solutions osmotic pressure is same, solute or solvent may not be same.
Colligative properties are observed when _____________.
(i) A nonvolatile solid is dissolved in a volatile liquid.
(ii) A nonvolatile liquid is dissolved in another volatile liquid.
(iii) A gas is dissolved in nonvolatile liquid.
(iv) A volatile liquid is dissolved in another volatile liquid.
This is a multiple choice answer as classified in NCERT Exemplar
(i) and (ii)
Colligative properties are observed when a non-volatile solid or liquid are dissolved in a volatile liquid.
Match the items given in Column I and Column I
|
Column I |
Column II |
|
(i) Saturated solution |
(a) Solution having same osmotic pressure at a given temperature as that of given solution. |
|
(ii) Binary solution |
(b) A solution whose osmotic pressure is less than that of another. |
|
(iii) Isotonic solution |
(c) Solution with two components. |
|
(iv) Hypotonic solution |
(d) A solution which contains maximum amount of solute that can be dissolved in a given amount of solvent at a given temperature |
|
(v) Solid solution |
(e) A solution whose osmotic pressure is more than that of another. |
|
(vi) Hypertonic solution |
(f) A solution in solid phase. |
This is a matching answer type question as classified in NCERT Exemplar
(i)- (d); (ii)- (c); (iii)- (a); (iv)- (b); (v)- (f); (vi)- (e)
Match the items given in Column I with the type of solutions given in Column II.
|
Column I |
Column II |
|
(i) Soda water |
(a) A solution of gas in solid |
|
(ii) Sugar solution |
(b) A solution of gas in gas |
|
(iii) German silver |
(c) A solution of solid in liquid |
|
(iv) Air |
(d) A solution of solid in solid |
|
(v) Hydrogen gas in palladium |
(e) A solution of gas in liquid |
|
(f) A solution of liquid in solid |
This is a matching answer type question as classified in NCERT Exemplar
(i)- (e); (ii)- (c); (iii)- (d); (iv)- (b); (v)- (a)
Match the laws given in Column I with expressions given in Column II.
|
Column I |
Column II |
|
(i) Raoult’s law |
(a) ΔTf=Kfm |
|
(ii) Henry’s law |
(b) π=CRT |
|
(iii) Elevation of boiling point |
(c) P=x1p10 +x2p20 |
|
(iv) Depression in freezing point |
(d) ΔTb=Kbm |
|
(v) Osmotic pressure |
(e) p= KH.x |
This is a matching answer type question as classified in NCERT Exemplar
(i)- (c); (ii)- (e); (iii)- (d); (iv)- (a); (v)- (b)
Match the terms given in Column I with expressions given in Column II.
|
Column I |
Column II |
|
(i) Mass percentage |
(a) (a) Number of moles of the solute component/Volume of solution in litres |
|
(ii) Volume percentage |
(b) Number of moles of a component / Total number of moles of all the components |
|
(iii) Mole fraction |
(c) Volume of the solute component in solution /Total volume of solution *100 |
|
(iv) Molality |
(d) Mass of the solute component in solution/Total mass of the solution *100 |
|
(v) Molarity |
(e) Number of moles of the solute components/Mass of solvent in kilograms |
This is a matching answer type question as classified in NCERT Exemplar
(i)- (d); (ii)- (c); (iii)- (b); (iv)- (e); (v)- (a)
In the following questions a statement of assertion followed by a a statement of reason is given. Choose the correct answer out of the following choices.
(i) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(ii) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(iii) Assertion is correct statement but reason is a wrong statement.
(iv) Assertion and reason both are incorrect statements.
(v) Assertion is wrong statement but reason is correct statement.
Assertion: Molarity of a solution in liquid state changes with temperature.
Reason: The volume of a solution changes with change in temperature.
(i) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation: (i) In terms of 'Molarity (M), ' the number of moles of the solute dissolved per litre of solution is the strength of a solution.
(ii) Because the volume of a liquid changes with temperature, the volume of a solution changes as well, while the number of moles of solute in it remains constant (or unchanged). As a result, the molarity of the solution would be affected.
Assertion: When methyl alcohol is added to water, boiling point of water increases.
Reason: When a volatile solute is added to a volatile solvent elevation in boiling point is observed.
This is a assertion and reason answer type question as classified in NCERT Exemplar
(iv) Assertion and reason both are incorrect statements.
Explanation: (i) Both methyl alcohol and water are volatile liquids that, when mixed together to produce a binary solution, have a higher vapour pressure than the separate pure components. The boiling point of water is reduced as a result of this.
(ii) When methyl alcohol is introduced to water, the A-B interaction (also known as the A-A or B-B interaction) occurs, resulting in a positive divergence from Raoult's rule. The boiling point decreases when a positive divergence from Raoult's law suggests an increase in vapour pressure.
Assertion: When NaCl is added to water a depression in freezing point is observed.
Reason: The lowering of vapour pressure of a solution causes depression in the freezing point.
This is a assertion and reason answer type question as classified in NCERT Exemplar
(i) Assertion and reason both are correct statements and reason is correct explanation for assertion.
Explanation: When a nonvolatile solute (such as NaCl) is added to water, a NaCl solution is created. When compared to pure water, the solution has a lower vapour pressure because there are less water molecules at the liquid's surface. A dip, or drop in the freezing point of water, is noticed as a result of this decrease in vapour pressure.
Assertion: When a solution is separated from the pure solvent by a semipermeable membrane, the solvent molecules pass through it from pure solvent side to the solution side.
Reason: Diffusion of solvent occurs from a region of high concentration solution to a region of low concentration solution.
This is a assertion and reason answer type question as classified in NCERT Exemplar
(ii) Assertion and reason both are correct statements but reason is not correct
Explanation for assertion.
Explanation: Assertion is correct statement but reason is a wrong statement because a semipermeable membrane allows solvent molecules to move through a lower concentration solution to a higher concentration solution, osmosis is the flow of solvent molecules from the solvent side to the solution side through a semipermeable membrane.
02 Oct 2025
02 Oct 2025
Commonly asked questions
Shown below are portions of orbital diagrams representing the ground state electron configuration of certain elements. If ‘x’ of them violates Pauli exclusion principle and ‘y’ of them violates Hund’s Rule then what is ‘x’ multiplies ‘y’?
The Pauli exclusion principle states that no two electrons in an atom can have the same four quantum numbers. In other words, only two electrons may exist in the same atomic orbital, and these electrons must have opposite spins. (a) and (f) violate the Pauli exclusion principle. Hund's rule states that the most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins. (b), (d) and (e) violate Hund's rule.
How many total moles of electrons are transferred when 1 mole of Cr2O7 2- oxidized Fe2+ to Fe3+ in acidic medium?
Kindly go through the answer
(6)
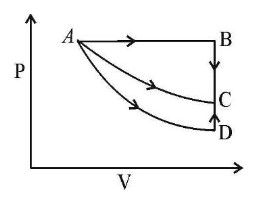
In the above diagram if a systems from initial state A (P1,V1,T1) goes to final state C (P2 ,V2 ,T1) from 3 different paths: Path 1 is A to C, Path 2 is A to B to C, Path 3 is A to D to C If 5 kJ of work is done by system in Path A to C then how much heat is transferred during Path A to D is:
(0) Path A to D is adiabatic.
Chemistry NCERT Exemplar Solutions Class 12th Chapter Two Exam