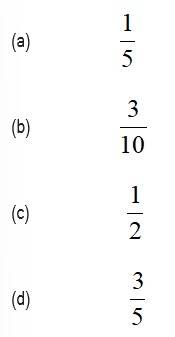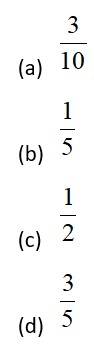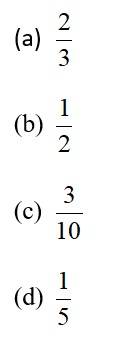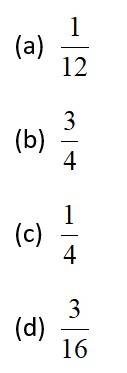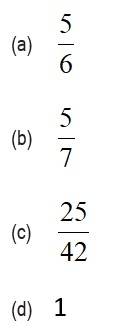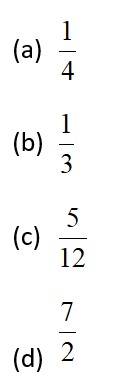Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
According to Bohr's postulates, in specific stable orbits, electrons revolve without emitting energy. Energy is only absorbed or emitted when electrons jump between these orbits, preventing them from spiraling into the nucleus.
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
The concept of quantized energy levels for electrons in atoms is introduced by Bohr's model. Although, Bohr's model could not explain the spectra of multi-electron atoms. It helped lay the foundation for quantum mechanics and successfully explained the spectral lines of hydrogen.
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
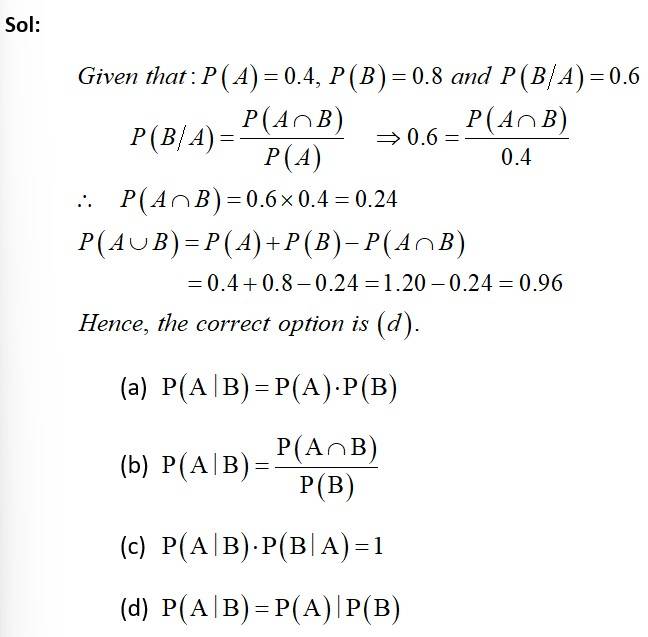
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
New answer posted
7 months agoContributor-Level 10
This is a Objective Type Questions as classified in NCERT Exemplar
Sol:
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers