Class 12th
Get insights from 12k questions on Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
Yes, PW Institute of Innovation, Lucknow accepts Class 12 marks for admission. The college offers BTech, BBA, and BVoc programmes at the UG levels. Candidates looking for course admission must complete the basic educational qualification, i.e., Class 12. The application process at the PW Institute of Innovation, Lucknow, is conducted online.
New answer posted
7 months agoContributor-Level 10
1. Cyclohexanone forms cyanohydrin in good yield because the ketonic group has very less steric hindrance at both the ortho position but 2,2,6 tri methyl cyclohexanone have high steric hindrance which reduces the attack from CN nucleophile.
New answer posted
7 months agoContributor-Level 10
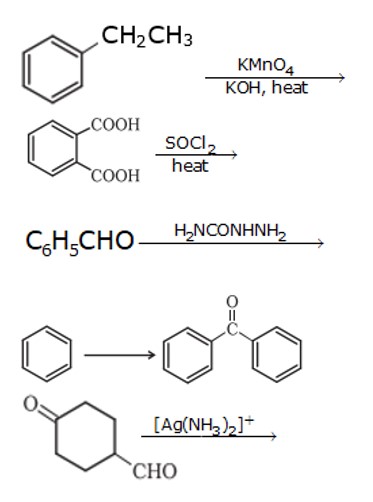
1. When 1- phenyl ethane is oxidized with a strong oxidizing agent like KMnO4, it forms a benzoic acid ion.
New question posted
7 months agoNew question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
1. Acetylation refers to the process of introducing an acetyl group (resulting in an acetoxy group) into a compound, namely the substitution of an acetyl group for an active hydrogen atom. A reaction involving the replacement of the hydrogen atom of a hydroxyl group with an acetyl group (CH3CO) yields a specific ester, the acetate. Acetic anhydride is commonly used as an acetylating agent reacting with free hydroxyl groups., this reaction is usually carried out in the presence of base like pyridine.

2. Aldeydes having no α-H undergo the disproportion reaction in the presence of Strong alkali, it is a chemical reacti
New answer posted
7 months agoContributor-Level 10
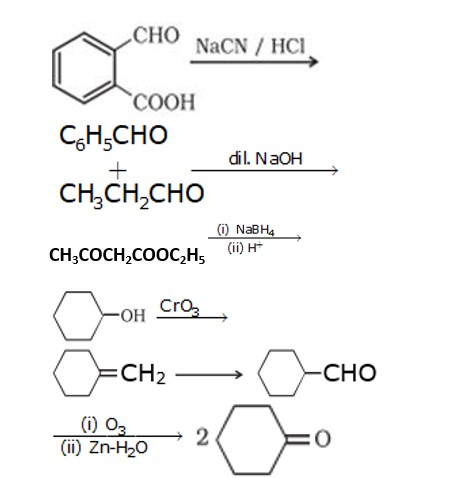
1. When propanone reacts with NaBH4 it will form propan-2-ol.This alcohol is dehydrated to form propene.
2. When benzoic acid is treated with SOCl2 it chlorinates the acid. After controlled hydrogenation it forms benzaldehyde
3. When ethanol is treated with Cu at 573 k, it will oxidize to ethanal. When ethanal is treated with Dil.NaOH it will form 3-Hydroxy butanal
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 684k Reviews
- 1800k Answers