Ncert Solutions Chemistry Class 12th
Get insights from 2.6k questions on Ncert Solutions Chemistry Class 12th, answered by students, alumni, and experts. You may also ask and answer any question you like about Ncert Solutions Chemistry Class 12th
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
Zn2+ has no unpaired electron and colourless in aqueous solution.
New answer posted
3 months agoContributor-Level 10
KCN
R – X + KCN
R – X + AgCN
KCN is ionic therefore ionised and attack occurs through carbon.
AgCN is covalent therefore attack starts with Nitrogen.
New answer posted
3 months agoContributor-Level 10
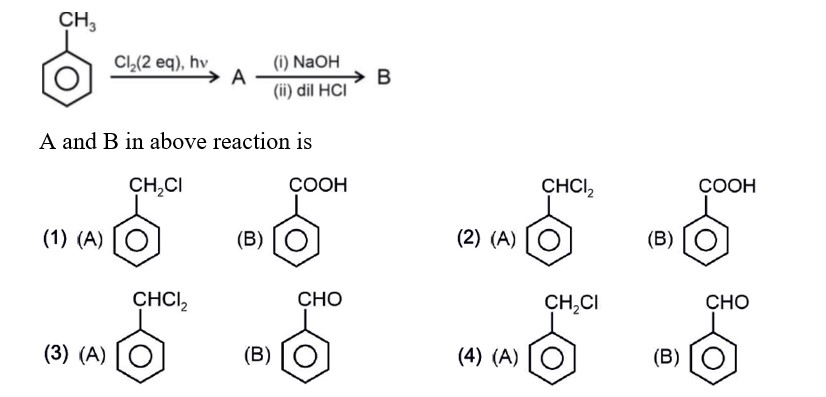
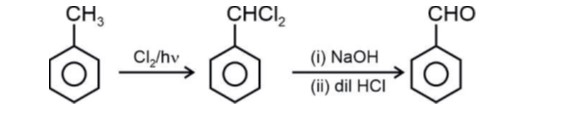
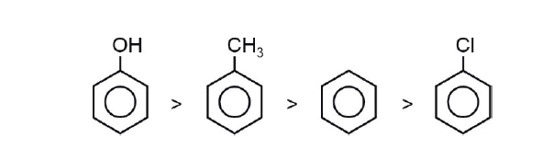
Order of reactivity towards electrophile
Strength of +M/+R : –OH > –CH3 > –Cl
In case of halogens, their –I effect dominates over
+M hence –Cl is deactivating and is lesser reactive than for incoming electrophile.
New answer posted
3 months agoContributor-Level 10
This is an example of chromyl chloride test
K2Cr2O7 + 4KCl + 6H2SO4? 6KHSO4 + 2CrO2Cl2 + 3H2O
Oxidation state of Cr is +6.
New answer posted
3 months agoContributor-Level 10
A, B and C are correct.
Mn2O7 is a green oil at room temperature.
V2O4 react with acids to give VO2+.
CrO is Basic and CrO3 is acidic.
V2O5 react with acids as well as alkali.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers