Some Basic Principles and Techniques
Get insights from 74 questions on Some Basic Principles and Techniques, answered by students, alumni, and experts. You may also ask and answer any question you like about Some Basic Principles and Techniques
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
28.
S.No. | Inductive Effect | Resonance Effect |
1 | It involves the displacement of σ electrons in saturated compounds. | It involves displacement of π electrons or Lone pair of electrons in unsaturated and conjugated compounds. |
2 | In inductive effect, there is slight displacement of σ electrons and partial positive or negative charge develops. | In this effect, there is complete transfer of π electrons and as a result, complete positive or negative charge develops. |
3 | Inductive effect can move only up to 3 to 4 carbons. | In this case, movement of electrons along the length a conjugated system takes place. |
New answer posted
9 months agoContributor-Level 10
(i) In this case CH3+ is the most stable species because replacement of H atom by Br atom (-Inductive effect) increases positive (ii
(ii)
![]() is most stable because negative charge on carbon atom is dispersed due to -I effect of Cl. More Cl atoms, more dispersal of negative charge and more stabilized.
is most stable because negative charge on carbon atom is dispersed due to -I effect of Cl. More Cl atoms, more dispersal of negative charge and more stabilized.
New answer posted
9 months agoContributor-Level 10
![]() is most stable because negative charge on carbon atom is dispersed due to -I effect of Cl. More Cl atoms, more dispersal of negative charge and more stabilized.
is most stable because negative charge on carbon atom is dispersed due to -I effect of Cl. More Cl atoms, more dispersal of negative charge and more stabilized.
New answer posted
9 months agoContributor-Level 10
27. In this case CH3+ is the most stable species because replacement of H atom by Br atom (-Inductive effect) increases positive charge on the carbon atom and destabilizes the species.
New answer posted
9 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
SO3 acts as an electrophile as in this, three highly electronegative oxygen atoms are attached to the sulphur atom which makes it electron deficient. It can be shown by resonance that it gets positive charge and acts as an electrophile.

New answer posted
9 months agoContributor-Level 10
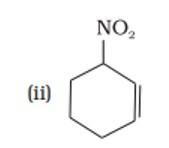
18. In this structure i.e., 3-nitrocyclohex-l-en (Carbon atoms of the ring are numbered in such a way that double bonded carbon gets the lowest number followed by the nitro group -NO2.
New answer posted
9 months agoContributor-Level 10
24. In this structure i.e., 3-ethyl, 4-methylhept-5-en-2-one. (the longest carbon chain is selected in such a way that the functional group > C = O gets the lowest possible locant.
New answer posted
9 months agoContributor-Level 10
23. Whenever an organic compound contains both N and S, then on fusion with Na metal compound gives NaSCN or NaCN and Na2S depending on the quantity of Na metal. If Na metal is less, then only NaSCN is formed. In that case L.E on treating with FeSO4 and H2SO4 gives red colour due to the formation of ferric thiocyanide Fe (SCN)3. In case of NaCN, L.E on treating with FeSO4 and H2SO4gives Prussian blue colour.
Chemical reactions: Fe2+ →Fe3+ (Oxidation)
Fe3+ + 3NaSCN →Fe (SCN)3 + 3Na+
&nb
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 687k Reviews
- 1800k Answers