Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
5.2
As Physisorption is Exothermic in nature, which means when gas gets adsorbed on the solid surface, Heat is evolved. So, according to Le-Chatelieres when the temperature is an increased reverse process (Desorption) will be favoured. So, Physisorption decreases with the increase of temperature.
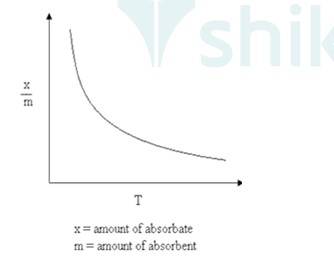
Where x/m: Volume of gas adsorbed
T: Temperature.
New answer posted
8 months agoContributor-Level 10
5.1
The two characteristics of Chemisorption are:
1. In Chemisorption which is highly specific in nature, the adsorb ate and adsorbent get attached by chemical bonds which are either covalent or ionic in
2. High activation energy is required and high temperature is also
3. Chemisorption increases with the increase in surface area which results in more number of active
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
