Surface Chemistry
Get insights from 216 questions on Surface Chemistry, answered by students, alumni, and experts. You may also ask and answer any question you like about Surface Chemistry
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (ii)
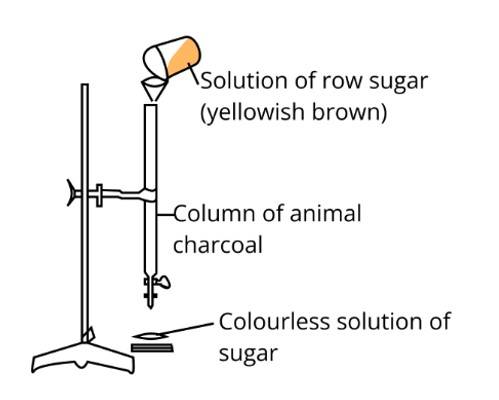
In the figure adsorption of coloured particle from charcoal is shown. Solution of raw sugar is filtered by animal charcoal
and yellowish brown colour of raw sugar is adsorbed and filterate is colourless which gives white colour on crystallization
hence the answer is (ii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct answer is (iv)
Charge on the sol particles can be a result of the following:
Due to electron capture by sol particles during electrodispersion of metals,
Due to preferential adsorption of ions from solution and/or
Due to formulation of electrical double layer.
Hence the correct answer is (iv)
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is iii.
River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in its deposition with the formation of delta hence the answeris (iii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct answer is (ii)
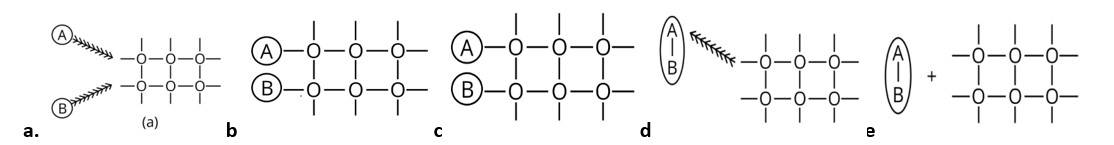
The correct sequence of steps involved in catalysis are:
(i) Adsorption of A and B on surface
(ii) Interaction between A and B to form intermediate
(iii) Starting of desorption from surface
(iv) Complete desorption from the surface
Therefore, the correct answer is (ii)
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (iv).
Colloidal particles are bigger aggregate than the number of particles in a colloidal solution hence, the values of
colligative are of small order as compared to values shown by true solutions at same concentration so the answer is (iv).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (iv).
Solid and liquid together forms up sol. In this case, solid is dispersed phase and liquid is the dispersion medium hence the answer is (iv).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (ii)
The rate of coagulation will be faster if the value of oppositely charge electrolyte is high hence the answer is (ii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (iv)
Peptisation is the process in which freshly prepared precipitate can be converted into colloidal solution so the answer is (iv).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (iii).
Lyophilic colloids have unique property of protecting lyophobic colloids. When a lyophilic sol is added to lyophobic solution, the lyophilic particles form a layer around the lyophobic particles and protect the latter from electrolytes. Lyophilic colloids used for this purpose are called protective colloids hence the answer is (iii).
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Type Questions as classified in NCERT Exemplar
Ans: Correct option is (ii).
Tyndall effect is defined as the optical property shown by colloidal particle. Above the critical micelle concentration, a solution of soap behaves as an associated colloid so it shows Tyndall effect hence the answer is (ii).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers