The S-block Elements
Get insights from 196 questions on The S-block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The S-block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
(i) 2Na + 2H2O → 2NaOH + H2
(ii) 2Na + O2 → Na2O2
(iii) Na2O2 + 2H20 → 2NaOH + H2O2
New answer posted
9 months agoContributor-Level 10
Sodium:
- Na+ions participate in the transmission of nerve signals, in regulating the flow of water across cell membranes.
- In the transport of sugars and amino acids into cell.
Potassium:
- K+ions activate many enzymes.
- Participate in the oxidation of glucose to produce ATP.
Magnesium:
- All enzymes that utilise ATP in phosphate transfer require magnesium as a cofactor.
- Mg is the central metal ion present in chlorophyll pigment in plants.
Calcium:
- Ca2+ ions are present in bones.
- plays important roles in neuromuscular function.
New answer posted
9 months agoContributor-Level 10
It is due to high lattice energy of LiF as compared to LiCl.
LiCl is soluble in water because its hydration energy is higher than its lattice energy.
New answer posted
9 months ago10.22. Why are lithium salts commonly hydrated and those of the other alkali ions usually anhydrous?
Contributor-Level 10
Li+ can polarize water molecules easily than the other alkali metal ions because of its small size.
New answer posted
9 months agoContributor-Level 10
Limestone:
- A raw material for cement.
- It is used as a building material in the form ofmarble and in the manufacture of quick lime.
- used in the manufactureof high quality paper. It is also used as anantacid, mildabrasive in tooth paste, aconstituent of chewing gum, and a filler incosmetics.
Cement:
- It is used in concrete and reinforcedconcrete, in plastering and in the construction
- of bridges, dams and buildings
Plaster of Paris:
- It is used in the building industry as well as plasters.
- It is usedfor immobilising the affected part of organ wherethere is a bone fracture or sprain.
- It is alsoemployed in dentistry, in ornamental work andfor making ca
New answer posted
9 months agoContributor-Level 10
Since group 1 hydroxides and carbonates due to large size contain higher hydration energy than the lattice energy so, they are easily soluble in water. Whereas, in magnesium and calcium due to small size their lattice energy dominates over hydration energy they are sparingly soluble in water.
New answer posted
9 months agoContributor-Level 10
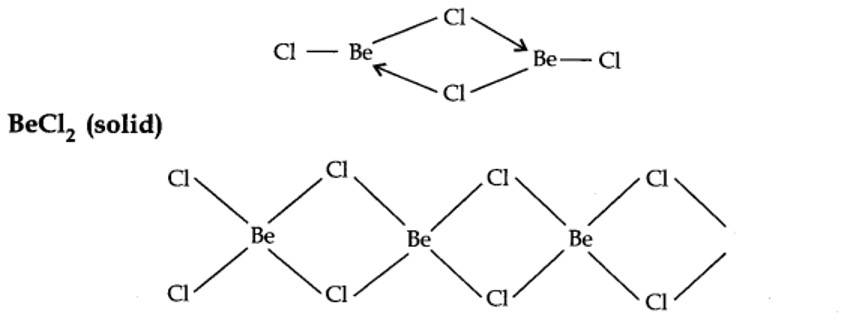
BeCl2 (vapour)
In the vapour state, it exists as a chloro-bridged dimer.
New answer posted
9 months agoContributor-Level 10
(i) Caustic soda
It is used in (a) the manufacture of soap, paper, artificial silk and a number of chemicals, (b) in petroleum refining, (c) in the purification of bauxite, (d) in the textile industries for mercerising cotton fabrics, (e) for the preparation of pure fats and oils, and (f) as a laboratory reagent.
(ii) Sodium carbonate
(a) It is used in water softening, launderingand cleaning.
(b) It is used in the manufacture of glass, soap, borax and caustic soda.
(c) It is used in paper, paints and textileindustries.
(d) It is an important laboratory reagent bothin qualitative and quantitative analysis
(iii) Quick lime
(a) It is an i
New answer posted
9 months agoContributor-Level 10
(i) Magnesium is burnt in air to form magnesium oxide and magnesium nitride.
2Mg + O2 → 2MgO
3Mg + N2 → Mg3N2.
(ii) Quick lime is heated with silica above 1273 K to obtain calcium silicate
CaO+SiO2 → CaSiO3.
(iii) Chlorine reacts with slaked lime to form calcium hypochlorite- a constituent of bleaching powder.
2Ca (OH)2 + 2Cl2 → CaCl2 + Ca (OCl)2 + 2H2O.
(iv) Calcium nitrate is heated to obtain CaO, NO2 and O2.
2Ca (NO3)2→2CaO+4NO2+O2.
New answer posted
9 months agoContributor-Level 10
(i) Sodium metal is manufactured by electrolysis of a fused mass of NaCl 40% and CaCl2 60% in Down's cell at 873 K, using iron as cathode and graphite as anode. Na is liberated at the cathode.
At cathode:
Na+ + e– → Na (l)
At anode:
2Cl– (melt) → Cl2 (g) + 2e–.
(ii) Sodium hydroxide is manufactured by electrolysis of an aqueous solution of NaCl (brine) in Castner-Kellner cell.
At cathode:
Na+ + e– → Na
2Na + Hg → Na – Hg + 2H2O
2Na- Hg + 2H2O → 2NaOH +H2 +Hg
At anode:
Cl– – e– → Cl
Cl + Cl→ Cl2
(iii) Sodium peroxide:
Sodium is heated in excess of oxygen to form sodi
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
