The S-block Elements
Get insights from 196 questions on The S-block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The S-block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
8 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct option (iv)
BaCO3 will be the most thermal stable. The size of Ba2+ is very large due to which it has low Polarising power and cannot polarise the oxygen atom. As the positive ions gets larger on moving down the group, the effect on the carbonate ion near them decreases. Thus, the carbonate ions of large cations are stable.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (iii)
Li due to its small size it has a high value of hydration enthalpy. Due to the high hydration enthalpy of Li atom, it is the strongest reducing agent in the aqueous medium.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (i)
The reactivity of alkali metals increases on moving down the group. So, Li is least reactive. It will react with water least vigorously.
New answer posted
8 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (iv)
In alkali metals, on going down the group, as the metallic strength decreases, melting point decreases. Thus, Cs has the lowest melting point and melts at 30°C.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
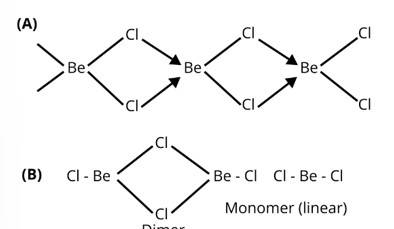
In the solid state BeCl2 has a polymeric chain like structure. Be atom is surrounded by four Cl atoms among which two of them are bonded through covalent bond and other two are through co-ordinate bond. Structure of BeCl2 is given by structure A.
In gaseous state beryllium chloride exists as dimer (Be2Cl4) which dissociates to the monomer at about 1200 K temperature which is linear in structure.
Structure of BeCl2 in gaseous state is given by the structure B.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The flame is due to the excitation of the electron from its higher energy state to the lower energy states. Due to the small atomic and ionic size of beryllium and magnesium, electrons are tightly bound to the atom. The electrons of Be and Mg does not gain excitation from the energy provided by the flame. Hence they do not show any flame in the flame test.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The lewis structure of O2– ion is,
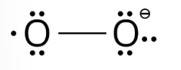
Oxygen atom having no charge has 6 electrons, so its oxidation number is zero. Oxygen atoms containing −1 charge have 7 electrons, so its oxidation number is −1. The average oxidation state of oxygen in this ion is, =1/2
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In the Solvay process, carbon dioxide is transferred through a concentrated solution of ammoniacontaining sodium chloride, which forms ammonium carbonate followed by ammonium hydrogen carbonate. The chemicals in ammonium hydrogen carbonate are different and are heated to form sodium carbonate. NH3 is found in a solution containing NH4Cl that is heated and treated with Ca (OH)2? The reaction of (NH4)2CO3 with NaCl provides two products, Na2CO3 and NH4Cl both soluble in water which do not shift to the right balance.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Ionic compounds are formed from the alkali metals due to their large ionic size and low ionization enthalpy. Thus, they are soluble in water. But, due to the small ionic size, high ionization enthalpy, and high electronegativity of lithium, it forms compounds of covalent nature and thus, are soluble in organic solvents.
New answer posted
8 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO4 and BaSO4 are insoluble The greater hydration enthalpy of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and therefore, their sulfates are soluble.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers
