The S Block Elements
Get insights from 95 questions on The S Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The S Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (iii)On moving down the group as the size increases, the nuclear charge attraction decreases. The effect of increasing size outweighs the increasing nuclear charge. Thus, ionization enthalpy decreases. The order of decreasing ionization enthalpy is,
Li>Na>K>Rb
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct option (i)
BeCl2 is covalent in nature due to the high polarising power of beryllium (+2) ion. On moving down the group, as the size increases, covalent nature decreases. Therefore, beryllium chloride being most covalent is soluble in ethanol.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct option (i)
As the size of the metal increases, the basic character of the hydroxide increases down the group. The solubility of hydroxide increases, and hydroxide of Be and Mg is almost insoluble.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct option (i)
Due to the smaller size of the cation Be2+, and larger size of the anion CO32? , BeCO3 is unstable in air. Therefore, it is kept in CO2 atmosphere to avoid decomposition.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct option (iv)
BaCO3 will be the most thermal stable. The size of Ba2+ is very large due to which it has low Polarising power and cannot polarise the oxygen atom. As the positive ions gets larger on moving down the group, the effect on the carbonate ion near them decreases. Thus, the carbonate ions of large cations are stable.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (iii)
Li due to its small size it has a high value of hydration enthalpy. Due to the high hydration enthalpy of Li atom, it is the strongest reducing agent in the aqueous medium.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (i)
The reactivity of alkali metals increases on moving down the group. So, Li is least reactive. It will react with water least vigorously.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice type question as classified in NCERT Exemplar
Correct Option (iv)
In alkali metals, on going down the group, as the metallic strength decreases, melting point decreases. Thus, Cs has the lowest melting point and melts at 30°C.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
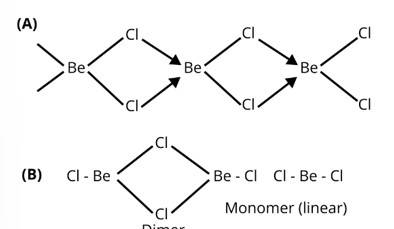
In the solid state BeCl2 has a polymeric chain like structure. Be atom is surrounded by four Cl atoms among which two of them are bonded through covalent bond and other two are through co-ordinate bond. Structure of BeCl2 is given by structure A.
In gaseous state beryllium chloride exists as dimer (Be2Cl4) which dissociates to the monomer at about 1200 K temperature which is linear in structure.
Structure of BeCl2 in gaseous state is given by the structure B.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The flame is due to the excitation of the electron from its higher energy state to the lower energy states. Due to the small atomic and ionic size of beryllium and magnesium, electrons are tightly bound to the atom. The electrons of Be and Mg does not gain excitation from the energy provided by the flame. Hence they do not show any flame in the flame test.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
