The S Block Elements
Get insights from 95 questions on The S Block Elements, answered by students, alumni, and experts. You may also ask and answer any question you like about The S Block Elements
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The lewis structure of O2– ion is,
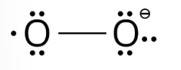
Oxygen atom having no charge has 6 electrons, so its oxidation number is zero. Oxygen atoms containing −1 charge have 7 electrons, so its oxidation number is −1. The average oxidation state of oxygen in this ion is, =1/2
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
In the Solvay process, carbon dioxide is transferred through a concentrated solution of ammoniacontaining sodium chloride, which forms ammonium carbonate followed by ammonium hydrogen carbonate. The chemicals in ammonium hydrogen carbonate are different and are heated to form sodium carbonate. NH3 is found in a solution containing NH4Cl that is heated and treated with Ca (OH)2? The reaction of (NH4)2CO3 with NaCl provides two products, Na2CO3 and NH4Cl both soluble in water which do not shift to the right balance.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Ionic compounds are formed from the alkali metals due to their large ionic size and low ionization enthalpy. Thus, they are soluble in water. But, due to the small ionic size, high ionization enthalpy, and high electronegativity of lithium, it forms compounds of covalent nature and thus, are soluble in organic solvents.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
BeSO4 and MgSO4 readily soluble in water while CaSO4, SrSO4 and BaSO4 are insoluble The greater hydration enthalpy of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and therefore, their sulfates are soluble.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(i) As the size of the cation increases, the thermal stability of carbonate increases. The more stable will be the oxide of an alkaline earth metal, the less stable will be the carbonate. Hence, beryllium carbonate will be highly unstable because its oxide will be stable.
(ii) Alkali metals and alkaline earth metals form oxides with oxygen and give metal oxides. The oxides are basic in nature. BeO is an exception because BeO is amphoteric.
They also react with water to form sparingly soluble hydroxides. On increasing the size of the cations beryllium oxide and magnesiu
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The element from group 2 is beryllium. Be (OH)2 is amphoteric. It reacts with both acids and bases. It reacts with acid to form beryllium chloride and it reacts with base to form beryllate ion which is soluble in sodium hydroxide. The reaction is shown below.
Be (OH)2+2OH−→ [Be (OH)4]2−
Be (OH)2+2HCl→BeCl2+2H2O
Beryllium sulfate are readily soluble in water.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
These two elements have similar properties because of their similar atomic and ionic radii.
(i) Both are lighter element and harder than the other metals in their respective groups.
(ii) The halides of both elements, LiCl and MgCl2 are soluble in ethanol.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
(i) O22−+ 2H2O → H2O2+2OH−
(ii) 2O2−+2H2O→2OH−+H2O2+O2
New answer posted
7 months agoWhen heated in air, the alkali metals form various oxides. Mention the oxides formed by Li, Na and K
Contributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Alkali metals forms oxide when reacted with air. Lithium forms monoxide, sodium forms peroxide and potassium forms superoxide.
4Li+O2→2Li2O
Na+O2→Na2O2
K+O2→KO2
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Lithium has the highest negative reduction potential value. Due to the small size of lithium it has the highest ionization enthalpy. Due to this, the reducing power of lithium is the highest in an aqueous solution.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
