Atoms
Get insights from 99 questions on Atoms, answered by students, alumni, and experts. You may also ask and answer any question you like about Atoms
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
5 months agoContributor-Level 10
E_K - E_L = hc/λ_Kα
E_K - E_L = (4.14*10? ¹? * 3*10? )/0.071*10? = 17500 eV = 17.5 keV.
E_L = E_K - 17.5 = 27.5 - 17.5 = 10 keV.
New answer posted
5 months agoContributor-Level 10
Energy of electron = 3 eV
It forms H atom in n=3 state. Energy released E = 3 - (-13.6/9) = 3 + 1.51 = 4.51 eV.
Photon Energy = 4.51 eV
Threshold energy = hc/λ = 12400eVÅ / 4000Å = 3.1 eV.
kE_max = 4.51 - 3.1 = 1.41 eV
New answer posted
5 months agoContributor-Level 10
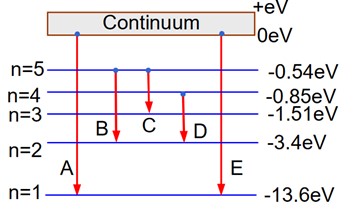
A : Series limit of Lyman series
B : Third line of Balmer series
C : Second line of Paschen series
New answer posted
5 months agoNew answer posted
5 months agoContributor-Level 10
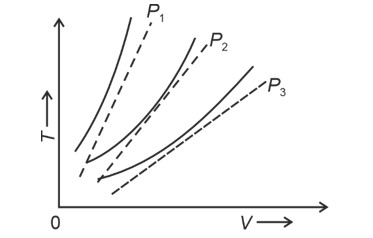
At same temperature, curve with higher volume corresponds to lower pressure.
(We draw a straight line parallel to volume axis to get this)
New answer posted
5 months agoContributor-Level 10
Statement I is true as atoms are electrically neutral because they contain equal number of positive and negative charges.
Statement II is wrong as atom of most of the elements are stable and emit characteristic spectrum. But this statement is not true for every atom.
New answer posted
6 months agoContributor-Level 10
According to de-Broglie's hypothesis
Cut-off wavelength of emitted X-ray =
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers