Chemical Equilibrium
Get insights from 80 questions on Chemical Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoThe volume (in mL) of 0.01M NaOH required to neutralise 20ml of 0.01M ortho-phosphoric acid is ____.
New answer posted
4 months agoContributor-Level 10
From Reaction
Δn? = 2 – 1 = 1
Kp = Kc (RT)^Δn?
Kp = Kc (RT)¹
Kc = Kp (RT)? ¹
New answer posted
4 months agoContributor-Level 10
All have same molarity, pH ∝ 1/ (Acidic strength) ∝ Basic strength
H? SO? → Acidic
NH? Cl (Salt of weak base and strong acid)
Acidic but less than H? SO?
NaCl → (Neutral solution)
NaOH → Basic (Strong Base)
So NaOH > NaCl > NH? Cl > H? SO?
New answer posted
4 months agoContributor-Level 10
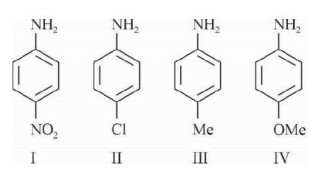
and basic strength ∝ 1/pK? ∝ K? ∝ +R ∝ 1/ (-R) ∝ +I ∝ 1/ (-I)
So basic strength order ⇒ IV > III > II > I, so pK? order is IV < III < II < I
New answer posted
4 months agoContributor-Level 10
Initially -> 1 mol -
At eq. 1-x mol 2x mol
Here; molecules of Cl2 = atoms of Cl
i.e moles of Cl2 = moles of Cl
So : 1 – x = 2x x = 1/3
Moles of Cl2 at equ
New answer posted
4 months agoContributor-Level 10
BCl → B? + Cl?
B? + H? O? BOH + H? , K? = K? /K?
0.25 – –
(0.25 – x) x
Given, pH = 2.7 = [H? ] = 2 * 10? ³
∴ x²/ (0.25) = (10? ¹? )/K?
= 4 * 10? * 4 * K? = 10? ¹?
= K? = (1/16) * 10? = 6.25 * 10? ¹?
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers