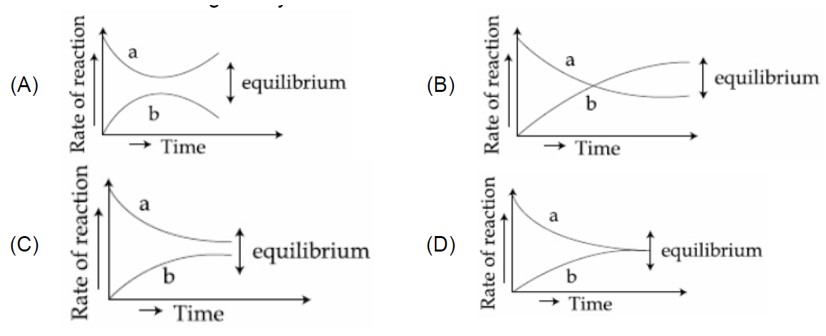
Chemical Equilibrium
Get insights from 80 questions on Chemical Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 9
Matching Processes with Catalysts:
o (a) Deacon's process: CuCl? used as catalyst
o (b) Contact process: V? O? used as catalyst
o (c) Cracking of hydrocarbon: ZSM-5 used as catalyst
o (d) Hydrogenation of vegetable oil: Particles Ni used as catalyst
New answer posted
4 months agoContributor-Level 10
In endothermic reaction formation of reactants is favoured upon decrease in temperature. Addition of inert gas at constant volume and temperature has no effect on equilibrium.
New answer posted
4 months agoContributor-Level 10
(x/m) = k (P)¹/?
log (x/m) = log k + (1/n) log P
Slope = 1/n = 2 So n = ½
Intercept ⇒ log k = 0.477 So k = Antilog (0.477) = 3
So (x/m) = k (P)¹/? = 3 [0.04]² = 48 * 10?
New answer posted
4 months agoNew answer posted
4 months agoContributor-Level 10
(b) It is harmful for trees and plants
(c) It causes breathing problem in human being and animals
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers