Chemical Equilibrium
Get insights from 80 questions on Chemical Equilibrium, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemical Equilibrium
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
When nucleoside is linked to phosphoric acid at 5 '-position of sugar moiety, we get nucleotide.
New answer posted
4 months agoContributor-Level 10
| List-I | | List-II |
| :- | :- | :- |
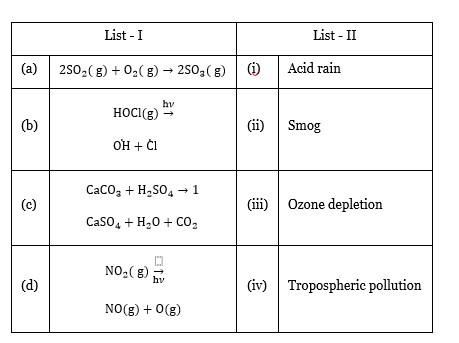
| (a) 2SO? (g) + O? (g) → 2SO? (g) | (iv) | Tropospheric pollution |
| (b) HOCl (g) - (hν)->? H +? l | (iii) | Ozone depletion |
| (c) CaCO? + H? SO? → | (i) CaSO? + H? O + CO? | Acid rain |
| (d) NO? (g) - (hν)-> NO (g) + O (g) | (ii) | Smog |
New answer posted
4 months agoContributor-Level 10
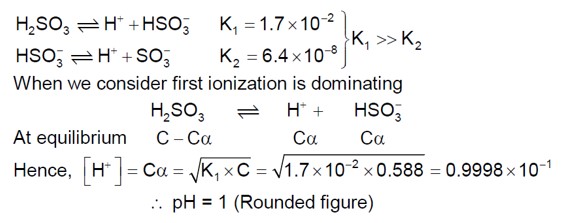
pH = pK? + log ( [CH? COONa] / [CH? COOH])
pH = 4.57 + log (0.1 / 0.01) = 5.57
New answer posted
4 months agoContributor-Level 9
ΔG° = -9.478 kJ/mol
Using ΔG° = -2.303 RT log K_p
-9.478 * 10³ = -2.303 * 8.314 * 495 log K_p
1 = log K_p ⇒ K_p = 10
Here, for the given reaction A (g)? B (g), K_p = K_c
Initial A = 22 mmol
At equilibrium A = 22 - x mmol; B = x mmol
K_c = [B] / [A] = (x/V) / (22-x)/V) = x / (22-x) = 10
x = 10 (22-x) ⇒ x = 220 - 10x ⇒ 11x = 220 ⇒ x = 20
So, mmol of B at equilibrium are 20.
New answer posted
4 months agoContributor-Level 10
Sucralose: Artificial sweetener
Glyceryl ester of stearic acid: The document incorrectly identifies this as "Sodium stearate which is synthetic detergent". Sodium stearate is a soap, not a synthetic detergent.
Sodium benzoate: Food preservative
Bithionol: Antiseptic
New answer posted
4 months agoContributor-Level 10
For the coagulation of a negative sol, the species Ba²? has the highest flocculating power (referring to the Hardy-Schulze rule, where higher charge leads to greater coagulation power).
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers