Chemistry Chemical Kinetics
Get insights from 111 questions on Chemistry Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
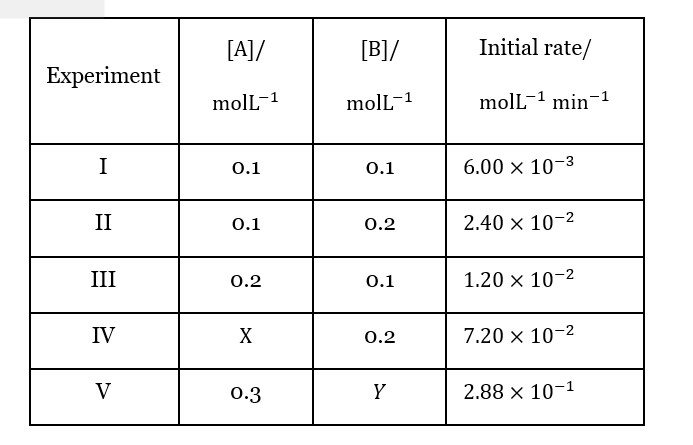
From I&II, rate∝ [B]². From I&III, rate∝ [A]¹.
From IV: 7.2e-2 = k (X) (0.2)². From II: 2.4e-2=k (0.1) (0.2)². X=0.3.
From V: 2.88e-1=k (0.3) (Y)². k=2.4e-2/ (0.1*0.04)=6.
2.88e-1 = 6 (0.3)Y². Y²=0.16. Y=0.4.
New answer posted
4 months agoContributor-Level 10
Successful collisions help in the formation of new products, whereas in unsuccessful collisions the molecules just bounce back without causing any reaction. In such cases, the reaction fails and no new product or bond is formed.
New answer posted
4 months agoContributor-Level 10
For a collision to happen, the molecules must collide with each other at a high frequency. But the result can still lead to disappointment. This is because even if molecules collide effectively, they also need to be aligned properly in symmetry. Only this can help in the formation of new bonds. Hence, the molecular orientation is considered important in he concept of collision theory.
New answer posted
4 months agoContributor-Level 10
Here are a few useful applications of this concept in our day to day lives:
- Preservation and Cooking of Food
- Chemical Manufacturing
- Pharmaceuticals
- Thermometer
- Biology Enzymes
- Electrical Appliances
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers