Chemistry Chemical Kinetics
Get insights from 111 questions on Chemistry Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
(4) C? H? O will have different alkyl group attached with polyvalent functional group that's why show metamerism
Only one arrangement possible so can not show metamerism.
(2) C? H? O → CH? – O – CH? – CH? Only one arrangement possible so can not show metamerism.
(1) No polyvalent functional group in C? H? , so can not show metamerism.
New answer posted
4 months agoContributor-Level 10
k = (2.303 / 5) log (0.1 / 0.001) = (2.303 * 2) / 5 = 4.606 / 5 = 0.921 min? ¹
New answer posted
4 months agoContributor-Level 10
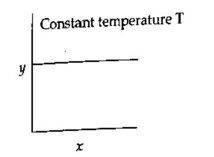
For a zero-order reaction Rate Vs conc graph will be straight line parallel to the x-axis.
For a 1st order reaction t? /? vs concentration will again be a straight line parallel to x-axis.
New answer posted
4 months agoContributor-Level 9
T? = 300 K; K? = 1 * 10? ³ s? ¹
T? = 200 K; K? =?
E_a = 11.488 kJ/mol
Using Arrhenius equation:
log (K? /K? ) = (E_a / 2.303R) * [ (T? - T? ) / (T? )]
log (K? / 10? ³) = (11.488 * 10³ / (2.303 * 8.314) * [ (-100) / (6 * 10? )]
log (K? / 10? ³) = -1
K? / 10? ³ = 10? ¹
K? = 10? s? ¹ or K? = 10 * 10? s? ¹
New answer posted
4 months agoContributor-Level 10
Using the integrated rate laws for both reactions and solving for time 't' yields a value of 108.
New answer posted
4 months agoContributor-Level 9
Half life, t? /? = 1min
Let, time of 99.9% completion of reaction be 't' min
Let the reaction is of first order
K = (2.303/t) log? ( [R]? / [R])
[R] = 0.001 [R]?
t = (2.303 * 3 min) / 0.693
t = 9.99 min
the nearest integer is 10.
New answer posted
4 months agoContributor-Level 9
For the reaction C? H? → C? H? + H? , calculate the enthalpy change (ΔH).
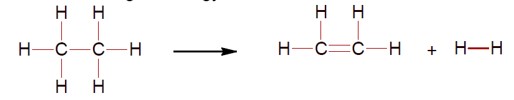
ΔH = [Bond energy (C-C) + 6 * Bond energy (C-H)] - [Bond energy (C=C) + 4 * Bond energy (C-H) + Bond energy (H-H)]
ΔH = 347 + 2 (414) - 611 - 436 = 128 kJ/mol.
New answer posted
4 months agoContributor-Level 10
The change in entropy for the following processes is negative (ΔS = -ve), indicating an increase in order:
Water (l) → Ice (s) at 0°C
H? O (l) → Ice (s) at -10°C
N? (g) + 3H? (g) → 2NH? (g)
Adsorption
New answer posted
4 months agoContributor-Level 10
Unimolecular Reactions might seem to not participate in the collision theory due to the presence of only a single molecule. In such cases, the molecule is activated by external forces such as heat, light, electricity, or by colliding with the walls of the container. This force charges the molecule enough to break the activation barrier and result in an effective collision.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers