Chemistry Chemical Kinetics
Get insights from 111 questions on Chemistry Chemical Kinetics, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Chemical Kinetics
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Endothermic reaction is a reaction which absorbs heat energy from the surrounding, making the environment cooler. The products have higher enthalpy than the reactants in this reaction.
On the other hand, Exothermic reaction is a reaction which releases heat energy into the surrounding, making the environment warmer. The products will have lower enthalpy than the reactants in this reaction.
New answer posted
4 months agoContributor-Level 10
This happens because although they have the same temeprature constant, some of their other properties can also vary such as activation energies, surface area of the reaction, concentration of the reactant, etc. As a result, the rate of a reaction comes out to be different for the reactions because of their dependence on these factors.
New answer posted
5 months agoContributor-Level 10
This is the type of reaction which bheavies like a first order reaction inspite of being a higher order reaction (second or third). This happens due to a particular reactant being present in an excessive quantity thorughout the chemical reaction.
New answer posted
5 months agoContributor-Level 10
These are the important highlights of integrated rate equations:
- predict the rate of a reaction
- calculate order of reaction (zero, first, second)
- understand mechanism of a reaction
- compute half life
- calculate the value of k
New answer posted
5 months agoContributor-Level 10
Yes, the phase of the reactant (solid, liquid or gas) actually affects the rate of a reaction. Gases and Liquids tend to move more freely and effectively as compared to solids, which increaes the frequency of particles colliding with each other.
New answer posted
5 months agoContributor-Level 10
This is possible because these catalysts have a lower activation energy, which allows a comparatively higher number of particles to react at a faster rate. More number of particles will mean more frequent collisions which speeds up the process.
New answer posted
5 months agoContributor-Level 10
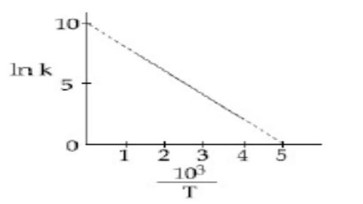
lnK = -E_a/RT + I
-E_a/R = slope is negative
⇒ -E_a/R = (10-0)/ (5-0)
E_a = 2R
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers