Chemistry Coordination Compounds
Get insights from 136 questions on Chemistry Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
3 months agoContributor-Level 10
[Ni (H2O)6]2+ Þ Ni2+ is octahedral and [Ni (CN)4]2– is square planar.
In [Ni (H2O)6]2+ Þ Ni2+ has two unpaired electrons and in [Ni (CN)4]2– Þ Ni2+ has no unpaired electrons. [Ni (H2O)6]2+ is coloured as it absorbs red light due to suitable d-d transition and complementary light emitted is green.
[Ni (CN)4]2– has strong field ligand so the electrons of Ni2+ pair up and it is colourless as it cannot absorb light from visible region.
New answer posted
3 months agoContributor-Level 10
(a) [Cr (H2O)6]+3 → Cr+3 →
(b) [Fe (H2O)6]+3 → Fe3+ →
(c) [Ni (H2O)6]+2 → Ni2+ →
(d) [V (H2O)6]+3 → V3+ →
New answer posted
3 months agoContributor-Level 10
Ni2+ : 4s03d8 (No pairing with Cl–)
[Ni (CO)4] : 4s03d10 (diamagnetic)
New answer posted
3 months agoContributor-Level 10
Correct order of increasing field strength of ligands is : I– < OH– < NCS–
New answer posted
3 months agoContributor-Level 10
EAN = Atomic No. – Oxidation state + 2 * CN
= 26 – 2 + 2 * 6
=36
New answer posted
3 months agoContributor-Level 10
[Co (NH3)6]3+, NH3 becomes strong ligand due to +3 oxidation state of cobalt ion. Hence electronic configuration of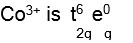
New answer posted
4 months agoContributor-Level 10
New answer posted
4 months agoContributor-Level 10
Correct order of increasing field strength of ligands is : I– < OH– < NCS–
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers

