Chemistry Coordination Compounds
Get insights from 136 questions on Chemistry Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
EAN = Atomic No. – Oxidation state + 2 * CN
= 26 – 2 + 2 * 6
= 36.
New answer posted
4 months agoContributor-Level 10
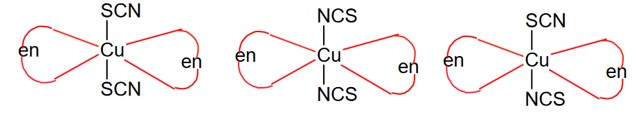
Cis (optically active) Trans (optically inactive)
d & l form (2) (1)
So, total 3 isomerism.
New answer posted
4 months agoContributor-Level 10
For precipitation of two moles of AgCl
Two Cl- will produce as a free anion
CoCl3.4NH3 -> complex will Cl (will not give 2Cl-)
complex will be H2 [PtCl6] will not any Cl-
will produce two Cl- ion.
precipitate formation
New answer posted
4 months agoContributor-Level 9
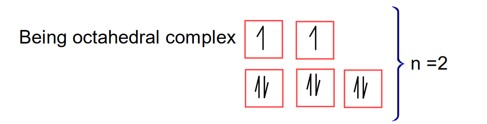
CN- is strong field ligand
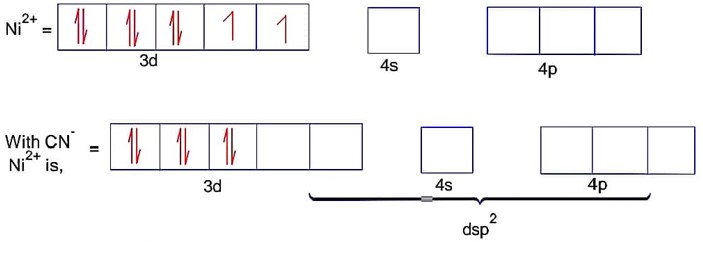
Here; is square planar and diamagnetic.
Ni = 4s23d8 Co is strong field ligand.
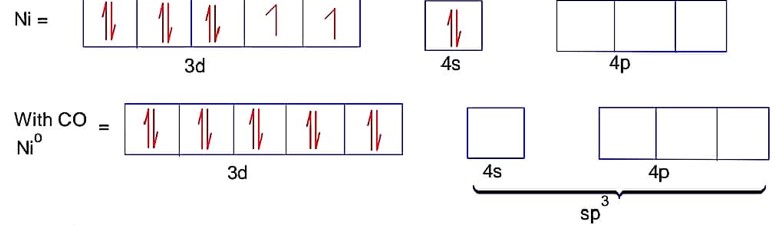
Here ; is tetrahedral and diamagnetic
has 3d8 configuration while has 3d10 configuration.
New answer posted
4 months agoContributor-Level 10
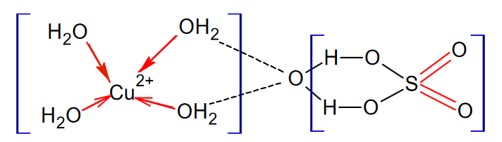
From the given structure of CuSO4 . 5H2O Cu (II) ion and oxygen bonds are present but ligands coordinating with Cu (II) ion are not O and S both.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers