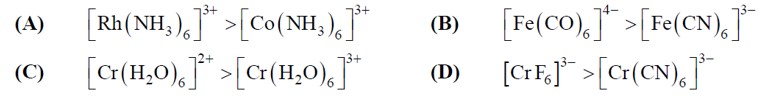Chemistry Coordination Compounds
Get insights from 136 questions on Chemistry Coordination Compounds, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Coordination Compounds
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
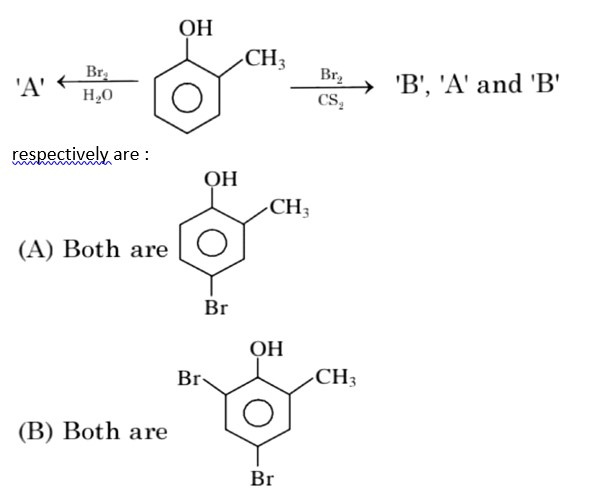
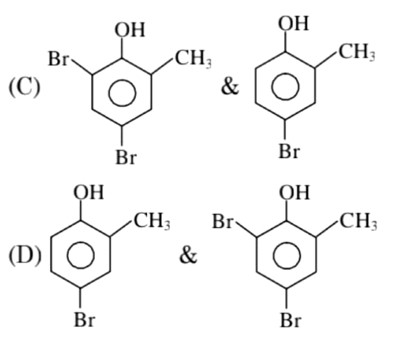
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
New answer posted
4 months agoContributor-Level 9
0.2 mole gives mole of (moles of )
1 mole complex gives moles
The correct answer should be option.
New answer posted
4 months agoContributor-Level 10
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
New answer posted
4 months agoContributor-Level 10
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
New answer posted
4 months agoContributor-Level 10
(a) [1rCl (CO) (PPh3)2] carbonylchloridobis (triphenylphosphine) iridium (I).
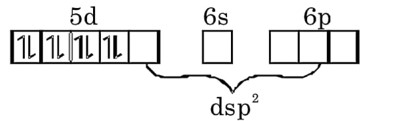
(b) Coordination number of Ir is four. Ir is in (+1) oxidation state with 4d8 configuration. It is trans isomer, so its geometry should be square planar.
(c) All electrons are paired, hence diamagnetic
(d) Can exhibit GI only.
New answer posted
4 months agoContributor-Level 10
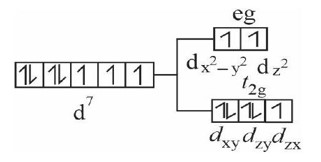
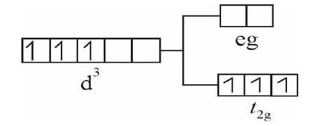
The given value of µ (spin only)
2.84 = √n (n + 2) BM, So, n = 2
Among the given configurations, d? system in strong field ligand will have 2-unpaired e? in t? g set of orbitals as shown below.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers