Chemistry Hydrocarbon
Get insights from 93 questions on Chemistry Hydrocarbon, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Hydrocarbon
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
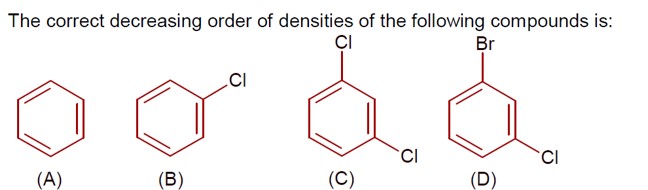
5 months agoContributor-Level 10
Density ∝ molar mass, as size of given molecules is nearly same
New answer posted
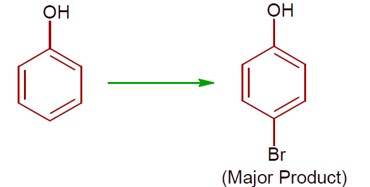
5 months agoContributor-Level 9
(a) Phenol with Br? / H? O gives 2,4,6-tribromophenol. (High ionisation due to polar solvent and high activation of ring)
(b) Phenol with Br? in CS? /CHCl? /FeBr? gives a mixture of o- and p-bromophenol, with p-bromophenol as the major product. (less ionisation due to non- polar solvent)
[Chemical reactions showing bromination of phenol under different conditions]
New answer posted
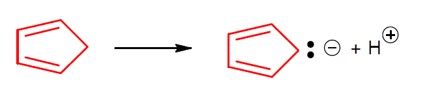
5 months agoContributor-Level 10
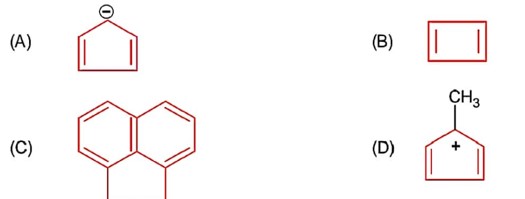
Conjugate base is highly stable.
Acidic strength ∝ stability of conjugate base.
So, 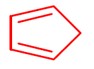
New answer posted
5 months agoContributor-Level 10
V 6V - -
- - 4V
Volume of CO2 = 4 *
Vx = 4V
x = 4
Volume of O2 = 6 *
y = 8
New answer posted
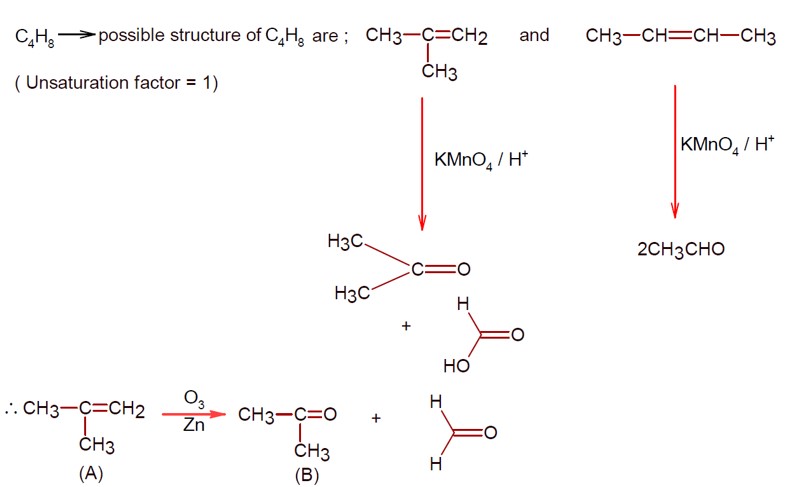
5 months agoContributor-Level 10
Hence
CH3 – CH2 – CHO can't be formed.
In case of unsymmetrical alkynes, more positive charge stabilizing carbon attacked by H2O and finally converted into carbonyl.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers