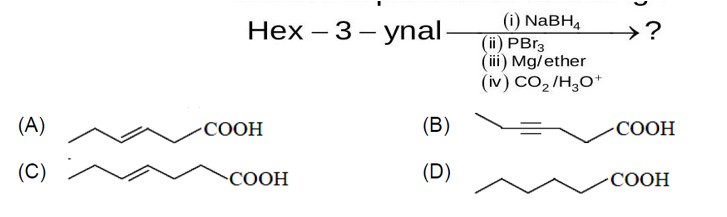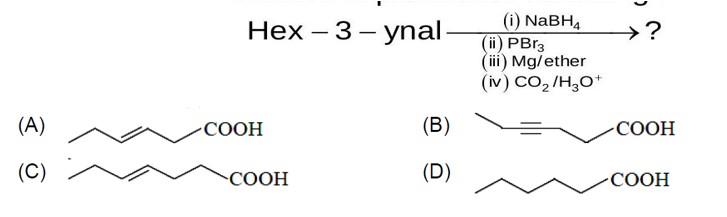Chemistry Hydrocarbon
Get insights from 93 questions on Chemistry Hydrocarbon, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry Hydrocarbon
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
4 months agoContributor-Level 10
Terminal alkynes and Non Terminal Alkynes are both variants of alkynes, but the difference lies in the position of the triple carbon bond. Terminal Alkynes have the triple carbon bond at the end of the chain, such as ethyne and propyne. On the other hand, Non Terminal Alkynes have their triple carbon bond in the middle of the chain. Example: 2-Butyene.
New answer posted
4 months agoContributor-Level 10
According to this rule, when a hydrogen halide (HX) or water is added to an asymmetrical alkene, the hydrogen atom (H) gets attached to the carbon atom of the double bond which has more hydrogen atoms, and the halide (X) or OH group attaches to the carbon atom which has fewer hydrogen atoms.
New answer posted
4 months agoContributor-Level 10
The most common method used for the identification of alkenes is the bromine water test. We simply have to add a few drops of bromine water to the compound which is to be identified. If the compound actually is an alkene, the reddish brown colour will get decolorized (? bond reacts with bromine and breaks the double bond).
New answer posted
4 months agoContributor-Level 10
Here are the important applications of alkanes in our everyday lives:
- Fuel in Vehicles
- Heating and Cooking
- Lubrication
- Power Generation
- Wax and Solvents
New answer posted
4 months agoContributor-Level 10
This happens due to the fact that they possess strong C-C and H-H bonds which are non-polar sigma bonds and cannot be broken easily. Also, they lack polar functional groups and are saturated hydrocarbons. These properties combined make them less reactive overall.
New question posted
5 months agoNew question posted
5 months agoNew question posted
5 months agoTaking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers