From LPG Cylinders in the kitchen to petrol in vehicles, hydrocarbons are everywhere. Hydrocarbons are present as the raw material for petroleum, natural gases, rubber, plastics, and in many materials used in daily life.
The detailed overview of Hydrocarbons helps you practice important definitions, reactions, and prepares you for the exams. The Hydrocarbons class 11th chemistry chapter 9 carries almost 10 marks out of 70, yet students fail to score. In this chapter overview, we’ve covered the Hydrocarbon NCERT notes, important questions, and reaction formulas that will help you to cover the important topic in a short time and score higher in board exams, as well as JEE and NEET.
Chemistry 11th Class Chapter 9 Revision Notes – Hydrocarbon covers the following topics:
- CLASSIFICATION OF HYDROCARBONS
- ALKANES
- ALKENES
- ALKYNES
- AROMATIC HYDROCARBON
- CARCINOGENICITY AND TOXICITY
If you want to clear your doubts about other chapters in chemistry, you can check the NCERT Solution Class 11 Chemistry.
- What are Hydrocarbons
- Classification of Hydrocarbons
- Alkanes
- Alkenes
- Alkynes
- Aromatic Hydrocarbons
- Carcinogenicity and Toxicity
- Hydrocarbons Chapter 9 Class 11th Important Formulas and Chemical Reactions
- FAQs
What are Hydrocarbons
As the name says, Hydrogen+Carbons (Hydrocarbons) are organic compounds made of only hydrogen (H) and Carbon (C) atoms.
Hydrocarbons can be of three types: Alkanes, Alkenes, and Alkynes.
Hydrocarbons are the main compounds of petroleum and natural gases, used as a fuel and raw material in various resources such as rubber, fuel, fabric, etc. They are highly combustible, releasing carbon dioxide and heat when burned.
Classification of Hydrocarbons
Hydrocarbons are classified in three categories: Saturated, Unsaturated, and Aromatic Hydrocarbons. The classification of hydrocarbons is based on the type of carbon bond present.
For example,
Saturated Hydrocarbons (Alkanes) have C-C and C-H single bonds.
Unsaturated Hydrocarbons have C-C multiple bonds such as double bonds, triple bonds, or both.
Aromatic Hydrocarbons are cyclic compounds, i.e., Benzene (C6H6).
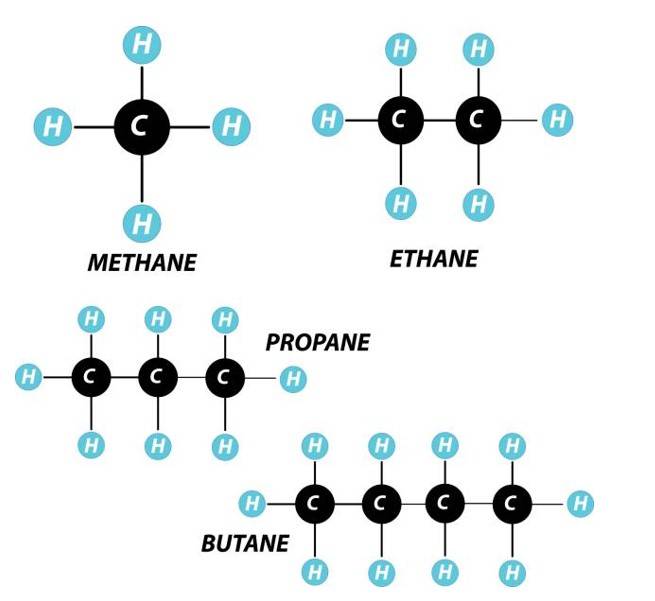
Alkanes
Alkanes are saturated open-chain compounds that contain only single bonds. The general formula for alkanes is CnH2n+2. Each carbon is sp3 hybridised with a bond angle of 109.5 degrees.
For example-
Methane, Ethane, and Propane
Alkenes
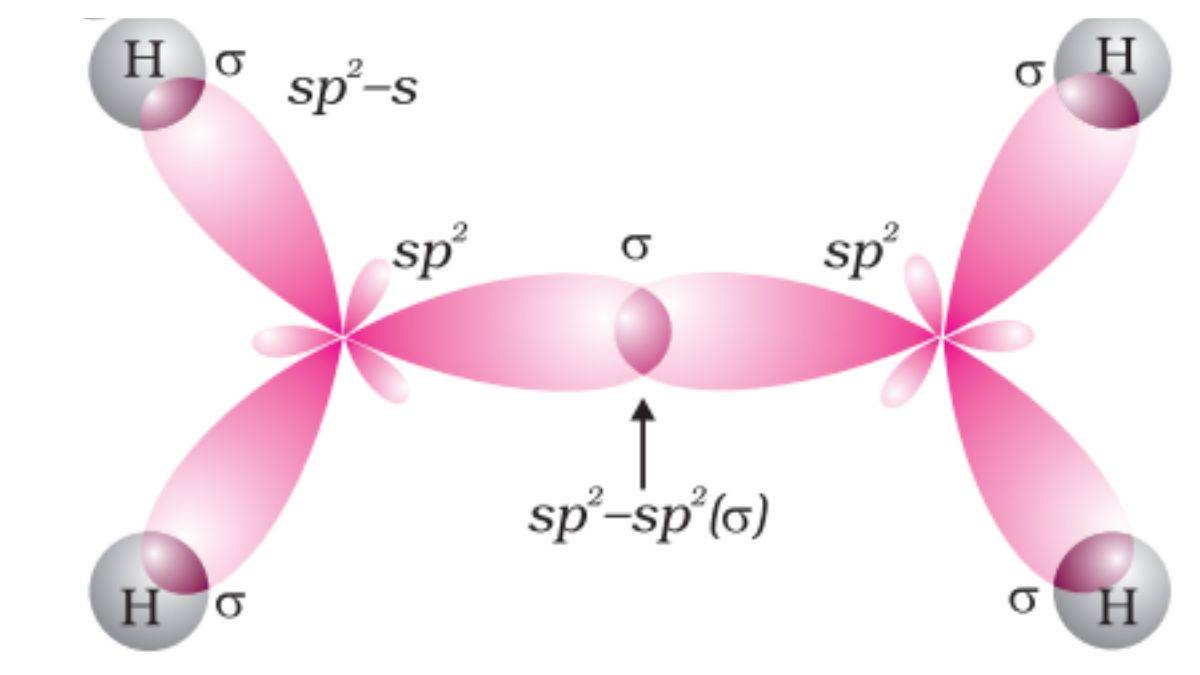
The general formula for alkenes is CnH2n. Alkenes are unsaturated hydrocarbons with a C=C double bond. Each carbon is sp2 hybridised with a bond angle of 120 degrees.
Alkynes
Alkynes are unsaturated hydrocarbons containing a Carbon-Carbon triple bond. The general formula is CnH2n-2. Each carbon of the triple bond is sp-hybridised, linear, 180°.
Physical Properties of Alkynes are similar of alkanes and alkenes, and the chemical properties are:
Addition reactions:
With hydrogen → alkenes → alkanes
With halogens/halogen acids → substituted alkenes/alkanes
Oxidation: Strong oxidising agents → acids or CO₂ + H₂O
Acidic nature: Terminal alkynes are acidic (react with Na, AgNO₃, Cu₂Cl₂).
Aromatic Hydrocarbons
C6H6 (Benzene) is an ideal example of Aromatic Hydrocarbons. The Hydrocarbons that contain a benzene ring and similar structures are Aromatic Hydrocarbons.
Benzene structure:
Molecular formula: C₆H₆
Planar, cyclic, conjugated system
All C-C bonds are equal in length due to resonance
Obeys Hückel’s rule (4n+2 π electrons, n=1 → 6 π electrons)
The chemical properties of Aromatic Hydrocarbons are:
Lectrophilic Substitution Reactions (EAS):
Nitration:
C₆H₆ + HNO₃ → C₆H₅NO₂ (with H₂SO₄)
Halogenation:
C₆H₆ + Cl₂ → C₆H₅Cl (FeCl₃ catalyst)
Sulphonation:
C₆H₆ + SO₃ → C₆H₅SO₃H (conc. H₂SO₄)
Friedel-Crafts alkylation:
C₆H₆ + CH₃Cl → C₆H₅CH₃ (AlCl₃ catalyst)
Side chain oxidation:
C₆H₅–CH₃ → C₆H₅–COOH (benzoic acid, by KMnO₄ oxidation).
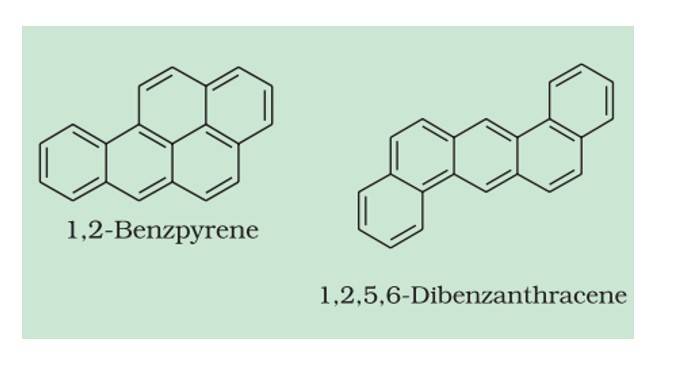
Carcinogenicity and Toxicity
Many polynuclear aromatic hydrocarbons (PAHs) with more than two benzene rings combine and become cancer-causing, such as benz[a]pyrene, benzanthracene, which are present in tobacco smoke, coal tar, and automobile exhaust. They are carcinogenic.
Hydrocarbons Chapter 9 Class 11th Important Formulas and Chemical Reactions
FAQs
Commonly asked questions
What are hydrocarbons in class 11th chemistry?
Hydrocarbons are organic compounds made of only carbon and hydrogen.
What are the four main hydrocarbons?
Alkanes, Alkenes, Alkynes, and Aromatics hydrocarbons are the four main hydrocarbons.
How to study Hydrocarbons for NEET?
To study Hydrocarbons for NEET, you can use the Hydrocarbons Class 11th NCERT solutions PDF.
Chemistry Hydrocarbon Exam
Student Forum
Other Topics under this Chapter
Other Class 11th Chemistry Chapters
- Chemistry Chemical Equilibrium
- Chemistry Structure of Atom
- Chemistry Redox Reactions
- Chemistry Some Basic Concepts of Chemistry
- Chemistry Organic Chemistry
- NCERT Class 11 Chemistry
- Chemistry Classification of Elements and Periodicity in Properties
- Chemistry Chemical Bonding and Molecular Structure
- Chemistry Hydrocarbon
- Chemistry Thermodynamics