Chemistry in Everyday Life
Get insights from 130 questions on Chemistry in Everyday Life, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry in Everyday Life
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
9 months agoContributor-Level 10
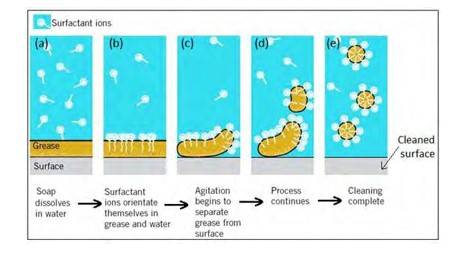
16.30 Soap molecules form micelles around an oil droplet [dirt] in such way that the hydrophobic parts of the stearate ions attach themselves to the oil droplet and the hydrophilic parts projects outside the oil droplet. Due to the polar nature of the hydrophilic parts, the stearate ions [along with the dirt] are pulled into the water, thereby removing the dirt from the cloth.
New answer posted
9 months agoContributor-Level 10
16.29
Soaps can be very easily used for checking the hardness of water as soap lathers with soft water and with hard water, soap is precipitated.
On the other hand, synthetic detergents are soluble in hard water and soft water. Hence it cannot be used for checking the hardness of water.
New answer posted
9 months agoContributor-Level 10
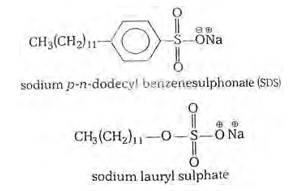
16.28
The commonly used soaps are the sodium and potassium salts of higher fatty acids. They are soluble in water and give good lather to it. On the other hand, calcium and magnesium salts of higher fatty acids (calcium and magnesium soaps) are insoluble in water and do not produce lather. Hard water contains Ca2+and Mg2+ ions. When a sodium or potassium soap is added to hard water, it gets converted into an insoluble calcium or magnesium soap as shown ahead.
Due to the conversion of sodium or potassium soaps into calcium or magnesium soaps in the presence of hard water, the common soaps are unable to emulsify the greasy dirt and c
New answer posted
9 months agoContributor-Level 10
16.27
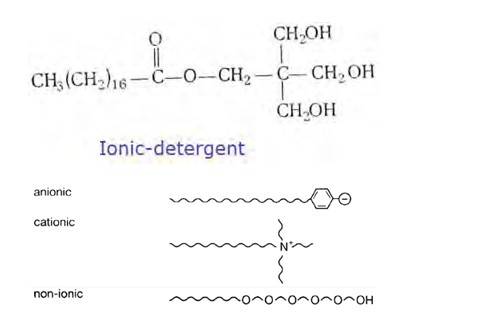
It has been found that the detergents having a great deal of branching in the hydrocarbon tail are not biodegradable and cause pollution in rivers and waterways. This is because the presence of side chains in the hydrocarbon tail stops bacteria from attacking and breaking the chains.
This results in slow degradation of detergent molecules leading to their accumulation in water ways. Alkylbenzene sulphonates having branched chain alkyl groups possess poor biodegradability and cause pollution in rivers and water Such detergents are termed as non-biodegradable or hard detergents.
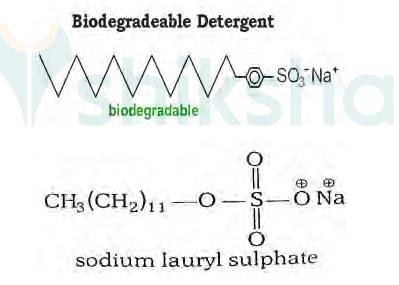
The alkyl sulphates do not possess branching and a
New answer posted
9 months agoContributor-Level 10
16.26
(i) Cationic detergents- Cationic detergents are quaternary ammonium salts [chlorides, bromides, acetates, etc.] having long chain alkyl groups. The cationic detergents are more expensive than anionic detergents and hence they find only limited use. However, they possess germicidal properties and are used quite extensively as germicides. The examples of cationic detergents are as follows:
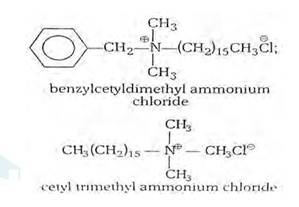
(ii) Anionic detergents- A detergent is said to be anionic when the large part of its molecule is an anion and is involved in the cleansing action. The anionic detergents are also effective in slightly acidic In slightly acidic solutions,
New answer posted
9 months agoContributor-Level 10
16.25
Synthetic detergents are superior cleansing agents as compared to soaps. This is due to the following reasons.
1. Detergents are soluble even in hard water. This is because of calcium and magnesium ions present in hard water form soluble salts with detergents. Hence, detergents can be used both in soft as well as in hard water. On the other hand, soaps form insoluble salts with calcium and magnesium ions and cannot be used in hard water.
2. The aqueous solutions of detergents are usually neutral. Therefore, they do not damage delicate fabrics and can be used for washing almost all types of On the other hand, aqueous solu
New answer posted
9 months agoContributor-Level 10
16.24
Alitame is a high potency artificial sweetener and it is not possible to control the sweetness imparted to food by alitame. Alitame is an artificial sweetener that is 2,000 times as sweet as sugar. This sweetness is very high as compared to the natural sugar and use of such sweetener is very critical while preparing sweet dishes. The structure of alitame is as follows:
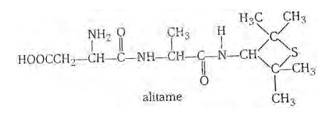
New answer posted
9 months agoContributor-Level 10
16.23
Artificial sweetening agents like Saccharin, Alitame, Sucrolose can be used in the preparation of sweets for a diabetic patient as they do not add any calories to the body.
Diabetic people are advised to consume low-calorie diet [fewer carbohydrates & more proteinaceous and fiber rich]. The refined sugar like sucrose adds calorie to the diet but complex sugar and starches do not add to the calorie intake of a person and at the same time impart a sweet taste to diet. Artificial sweeteners are generally either complex sugar or protein in nature
New answer posted
9 months agoContributor-Level 10
16.22
Artificial sweetening agents refer to those compounds which impart sweet taste to any food product but at the same, they do not add any calories to the body. Example: Aspartame, Alitame, Saccharin etc.
New answer posted
9 months agoContributor-Level 10
16.21
At elevated temperatures, aspartame is unstable and break down to give a tasteless compound because of which aspartame is limited to cold foods and drinks.
Aspartame is methyl ester of the dipeptide obtained from phenylalanine and aspartic acid. It is about 180 times as sweet as cane sugar. The structure of aspartame is as follows:
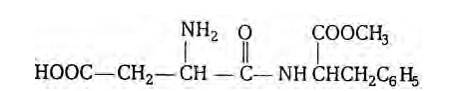
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 686k Reviews
- 1800k Answers