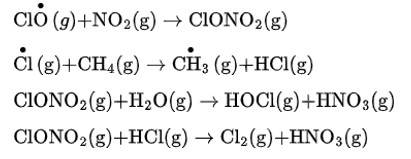Chemistry NCERT Exemplar Solutions Class 11th Chapter Fourteen
Get insights from 72 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Fourteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Fourteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Option (ii)
i.e., Both A and R are correct, but R is not the correct explanation of A is the answer the pH of rainwater is 5.6 because of the presence of hydronium ions produced due to the dissolution of carbon dioxide in rainwater from atmosphere and when the pH of rainwater drops from 5.6, it is being referred as acid rain. This can be shown as-
H2O (l)+CO2 (g)→H2CO3 (aq)
H2CO3 (aq)→H+ (aq)+HCO3− (aq)
New answer posted
7 months agoContributor-Level 10
This is a Assertion Type Questions as classified in NCERT Exemplar
Option (iii)
i.e., Both A and R are not correct is the answer since in cold places, sunlight required to grow plants is less. Due to which plants are kept in a glass house so that sunlight enters in the greenhouse to heat up the soil and plants. The warm soil and plants emit infrared radiations which are partially absorbed and partially reflected by the glass.
New answer posted
7 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Excessive amount of sulphate (i.e., greater than 500 ppm) in drinking water causes laxative effect, otherwise at moderate levels it is harmless.
New answer posted
7 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Scientists working in the Antarctica region reported that depletion of the ozone layer is commonly termed as an ozone hole over the South Pole in the Antarctic region. It was observed that a unique set of conditions were responsible for the ozone hole. In summer, nitrogen dioxide and methane react with chlorine monoxide and chlorine atoms forming chlorine sinks, preventing much ozone depletion, whereas in winter, special types of clouds called polar stratospheric clouds are formed over Antarctica. These polar stratospheric clouds provide a surface on which chlor
New answer posted
7 months agoContributor-Level 10
This is a Short answer type Questions as classified in NCERT Exemplar
Ozone is found to be thermodynamically unstable and undergoes decomposition into molecular oxygen and a dynamic equilibrium exists between the production and decomposition of ozone. The reactions involved can be shown as-
O2 (g) ![]() O (g) + O (g)
O (g) + O (g)
O (g) + O2 (g) +M![]() O3 (g) + M
O3 (g) + M
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i) the reaction is endothermic and requires very high temperature is correct since dinitrogen and dioxygen are the main constituents of air. These gases do not react with each other at a normal temperature. The dissociation energy of N2is very high due to the presence of triple bond and it is very stable.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (ii) Troposphere is correct since the lowest region of atmosphere in which the human beings along with other organisms live is called troposphere. It extends up to the height of approximately 10 km from sea level.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (ii) i.e., It has low concentration of oxidizing agents is the answer since the common components of photochemical smog are ozone, nitric oxide, acrolein, formaldehyde and peroxyacetyl nitrate (PAN). Photochemical smog causes serious health problems and both ozone and PAN act as powerful eye irritants. Photochemical smog contains high concentrations of oxidants.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (iii)
i.e., Decrease in the amount of dissolved oxygen in water is the answer since organic waste is oxidized by microorganisms in the presence of dissolved oxygen. Hence, oxygen decreases in water as a result it is harmful for aquatic life.
New answer posted
7 months agoContributor-Level 10
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (i) i.e., Ozone is not responsible for greenhouse effect is the answer since O3 is responsible for the greenhouse effect, its contribution is about 8% to 10%. About 75% of the solar energy reaching the earth is absorbed by the earth's surface, which increases its temperature. The rest of the heat radiates back to the atmosphere while some of the heat is trapped by gases such as carbon dioxide, methane, ozone, chlorofluorocarbon compounds (CFCs) and water vapour in the atmosphere. Thus, they add to the heating of the atmosphere. This is the reason behind glo
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers