Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen
Get insights from 59 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) The electronegativity of sp hybridization is higher than that of sp3 hybridization.
The +I effect destabilizes the carbanion.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(iii) The addition of alkyl halides to propene depends upon the H-X bond strength i.e. lesser the bond strength higher will be the reactivity of the alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
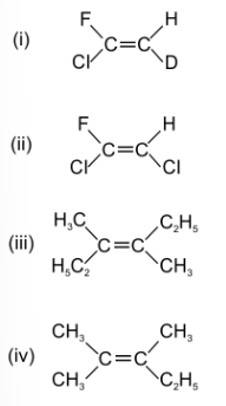
(iv) The two substituents attached to the same C atom of the C=C bond should be different to exhibit geometrical isomerisms.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) The reaction follows Markovnikov's rule leading to giving 2o carbocation as an intermediate.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
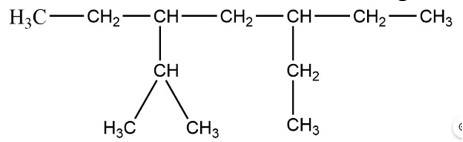
(i) 3, 6 – Diethyl – 2 – methyl octane
Octane is the longest chain here and the side chain follows the lowest sum rule.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(ii) R–Cl< RBr < RI
The reduction of alkyl halides depends upon the C-X bond strength i.e. lesser the bond strength higher will be the reactivity of alkyl halide. As down the group size of the halogens increases thus the bond strength decreases.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
(i) I2< Br2< Cl2< F2
The reactivity of halogens with alkanes depends on the electronegativity i.e. higher the electronegativity higher would be the reaction. As down the group reactivity decrease so thus its reactivity.
New answer posted
7 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
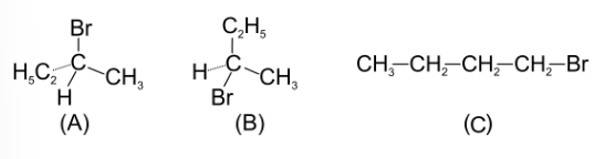
(iv) C > B > D > A
The boiling point increases with the increase in the length of the side chain. However, with the branching, the boiling point decreases due to the decrease in the surface area.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The reaction is
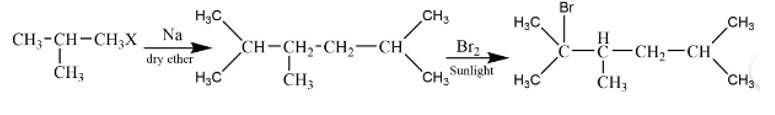
The alkane is
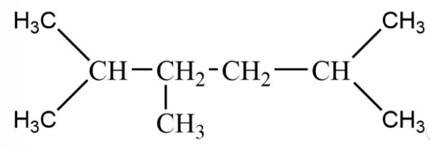
The tertiary bromide is
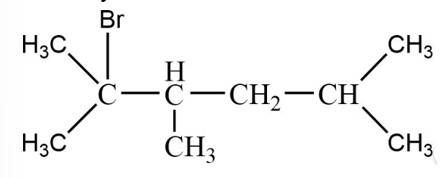
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The 2-methylpropane would lead to two types of radicals.
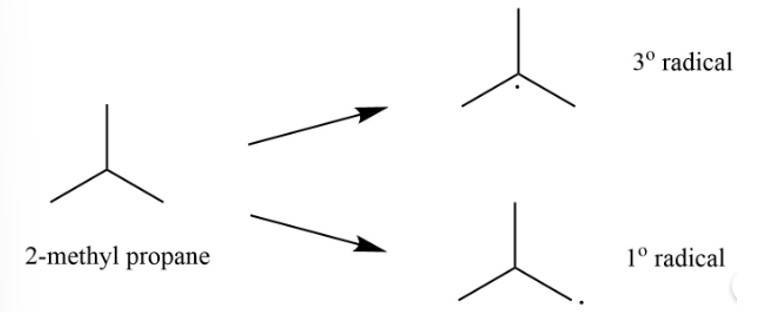
Among the two 3o radical is most stable as it contains 9 ∝ -hydrogen whereas 1o radical contains only 1 ∝ -hydrogen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers