Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen
Get insights from 59 questions on Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 11th Chapter Thirteen
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The rate-determining step involved in the reaction is
CH3-CH=CH2 + HX → CH3-CH+-CH3 + X-
So the rate-determining step depends on the bond energy of HX i.e. higher the bond energy lesser would be reactivity.
Thus the order of reactivity of these halogen acids is
HI>HBr>HCl
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The rotation around C-C single bond is possible but it is not completely free due to the torsional strain which is about 1-20 KJ mol-1.
New answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
Yes, it will execute geometrical isomerism as the product formed can be either cis-2-butene or trans-2-butene.
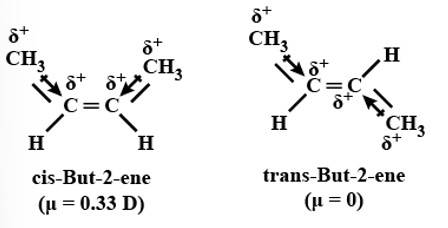
New question posted
7 months agoNew answer posted
7 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
If arenes would undergo electrophilic addition reaction then they would lose their aromaticity which would lead to comparatively less stability. However, alkenes undergo electrophilic addition reaction via carbocation intermediate.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
The bond energy of HCl is higher than that of HBr thus it is not cleaved by free radical mechanism to exhibit peroxide effect. However in case of HI the bond energy is so low that the iodine radical forms readily and after formation it combines to form an iodine molecule.
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
The structure of A is
The reaction involved are as follows:
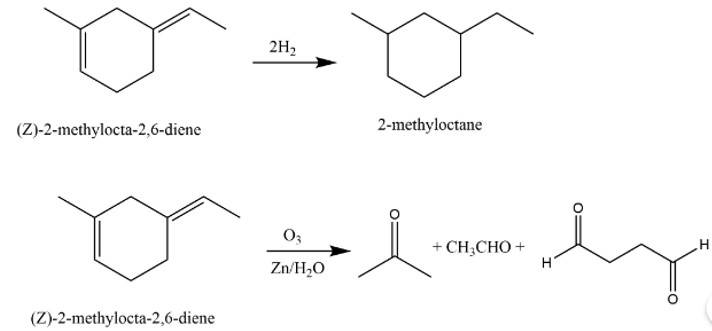
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
The molar mass of the compound = 3.38 x 22400/896 =831gmol-1
Element | Percentage | At.mass | Relative ratio | the simplest ratio |
C | 87.8% | 12 | 7.31 | 3 |
H | 12.19% | 1 | 12.19 | 5 |
Thus the empirical formula of the compound is C3H5
Molecular formula =n x empirical formula = 831/41 x C3H5 = C6H10
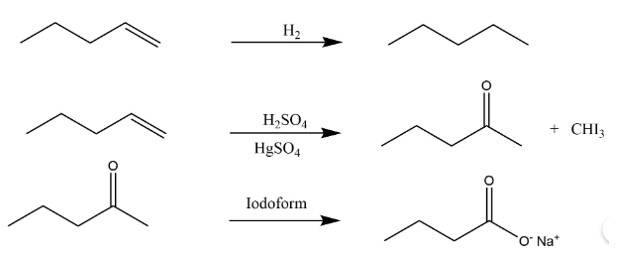
New answer posted
7 months agoContributor-Level 10
This is a long answer type question as classified in NCERT Exemplar
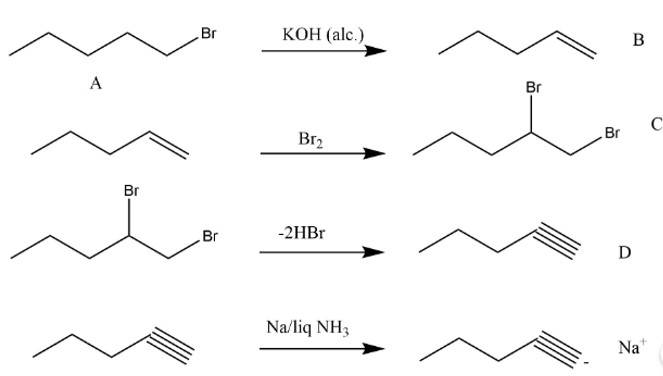
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
