Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Get insights from 115 questions on Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven, answered by students, alumni, and experts. You may also ask and answer any question you like about Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven
Follow Ask QuestionQuestions
Discussions
Active Users
Followers
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
When heated with concentrated NaOH in an inert CO2 environment. It formsPH3
P4 + 3NaOH + 3H2O→PH3 + 3NaH2PO2
As we progress from the top to the bottom in a group, the basic character of hydrides reduces PH3 is basic than NH3.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iv)
The E-H bond dissociation energy of SbH3 is the lowest among the aforementioned compounds, it easily releases H, making it a strong reducing agent. The greater the lowering agent, the weaker the E-H bond.
New question posted
6 months agoNew answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (i)
Fluorine is the most reactive element, forming hydrogen fluoride when it combines with hydrogen gas.
Due to the shorter bond length between the two atoms, it takes a lot of energy to break the link between hydrogen and fluorine.
As a result, as we move down the group, the bond dissociation energy drops.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (i)
The compound has the following number of electrons:
NO3- = 32e-
CO32- = 32e-
ClO3- = 42e-
SO3 - = 42e-
Hence, NO3- and CO32- are isoelectronic
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (iii)
If an electron pair is donated to a vacant orbital on one atom and a lone pair of electrons on the other, the bonding is designated pπ-dπ depending on the orbital to which the electron pair is donated and the orbital from which the electron pair is donated.
Because their valence shells lack d-orbitals, nitrogen, carbon, and boron cannot form a pi bond. Phosphorus, on the other hand, can produce pi bonds.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
H3PO2, H3PO4, H3PO4, and other oxoacids are formed by phosphorus. P is tetrahedrally surrounded by other atoms in phosphorus oxoacids. At least one P-H bond and an O-H bond are known to form in all of them.
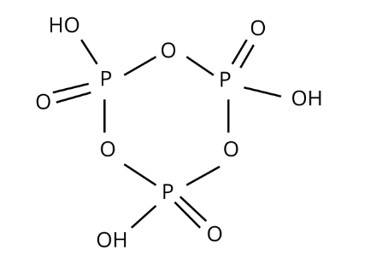
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option (ii)
When H2S is passed through an aqueous solution of salt acidified with dil. HCl in qualitative analysis, a black precipitate is produced. When the precipitate is boiled with dil. HNO3, a blue solution results. When an excess of ammonia aqueous solution is added to this solution, it produces a deep blue [Cu (NH3)4]2+ solution.
New answer posted
6 months agoContributor-Level 10
This is a multiple choice answer as classified in NCERT Exemplar
Correct option is (iii)
Hydrogen iodide is a more powerful reducing agent than sulphuric acid, it reduces the amount of iodine in the solution from H2SO4 to SO2 and HI to I2
When chloride salts are treated with sulfuric acid, HCl gas is formed, which produces a colourless gas.
NaCl + H2SO4→HCl + Na2SO4
Violet fumes are produced during the reaction due to the creation of iodine (I2) gas.
2NaI + H2SO4→Na2SO4 + 2HI ![]() 2H2O + SO2 + I2
2H2O + SO2 + I2
New answer posted
6 months agoContributor-Level 10
This is a short answer type question as classified in NCERT Exemplar
The following reaction with silver nitrate demonstrates phosphinic acid reducing behaviour:
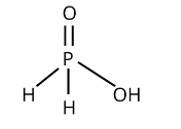
4AgNO3 + 2H2O + H3PO2→4Ag + 4HNO3 + H3PO4
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers
